

Hydrogen is the simplest and most abundant element in the universe(1). It is a basic building block in star formation(2) and ever-present in water, organic molecules and the Earth’s atmosphere. The gas is highly flammable and burns together with oxygen to form water. This key property explains the element’s name: the term hydrogen is derived from the Greek words for ‘creator’ and ‘water‘. The first scientific encounter with hydrogen took place in the late 16th century. However, the gas was confused with other flammable gases. Finally, in 1766 the chemist and physicist Henry Cavendish recognised hydrogen gas as an independent substance. He named it ‘flammable air’. Nowadays, hydrogen is widely used in the chemical industry, for example in the production of ammonia(1). Hydrogen is used as a low carbon fuel, particularly for heat; but also for hydrogen vehicles, seasonal energy storage and long distance transport of energy(4).
Researchers at Radboud University have discovered microorganisms that can live on the energy of hydrogen gas. As Prof. dr. Huub op den Camp explains: “These 'Knallgas' microbes only need hydrogen, oxygen, carbon dioxide and some minerals to grow. In addition, they are so efficient that they can even absorb atmospheric concentrations of hydrogen (0.53 ppm)”.
This group of hydrogenase (enzymes that catalyse the oxidation of H2) could be a strong controlling factor in the global H2 cycle1. The methanotroph Methylacidiphilum fumariolicum SolV, isolated from a volcanic mud pot, is thus far the only known methanotroph organism that can also rapidly grow on H2 as sole energy source, as well as oxidize subatmospheric H2(5). This not only implies the positive contribution these organisms can have in global warming (as methane-eating organisms), but also opens up a whole range of possibilities in creating a truly circular hydrogen economy.
Research on methane- and methanol eating bacteria, specifically methanol dehydrogenase, plays an important role in the Master’s course Genomics of Health and Envrironment, a collaboration between the departments of Molecular Biology, Human Genetics and Microbiology. Several Master’s internships provide opportunities to join this research, or co-author important scientific articles.
Sources
(1) hydrogen | Properties, Uses, & Facts. (2020, 1 June). Encyclopedia Britannica. https://www.britannica.com/science/hydrogen
(2) What’s in the Milky Way? (2019, 1 February). Curious. https://www.science.org.au/curious/space-time/whats-milky-way
(3) Wikipedia contributors. (2020a,24 July). Hydrogen. Wikipedia. https://en.wikipedia.org/wiki/Hydrogen
(4) Wikipedia contributors. (2020, 25 August). Hydrogen economy. Wikipedia. https://en.wikipedia.org/wiki/Hydrogen_economy
(5) Schmitz, R. A., Pol, A., Mohammadi, S. S., Hogendoorn, C., van Gelder, A. H., Jetten, M. S. M., Daumann, L. J., & Op den Camp, H. J. M. (2020). The thermoacidophilic methanotroph Methylacidiphilum fumariolicum SolV oxidizes subatmospheric H2 with a high-affinity, membrane-associated [NiFe] hydrogenase. The ISME Journal, 14(5), 1223–1232. https://doi.org/10.1038/s41396-020-0609-3
Helium is the smallest of all elements: its electrons are in a tight orbit due to the highly charged core(1). Helium is an inert, non-reactive, colourless gas and the second most abundant element in the universe. Helium molecules are mostly found in natural gas fields. It is the lightest noble gas, and widely known as a balloon filling. Because helium has the lowest melting and boiling point of all elements, it is an especially suitable coolant for many applications that require extremely low temperatures, such as superconducting magnets (e.g. in NMR and MRI equipment), cryogenic research and nuclear reactors(2). However, helium is becoming increasingly scarce, as the demand for natural gas (LNG) is declining.
Helium is used in research at the HFML-FELIX at Radboud University. Sanne Kristensen, PhD student Physics, examines fundamental properties of materials, especially the behaviour of electrons in materials under the influence of a strong magnetic field. “Unfortunately, the temperature of the environment around the research setup disturbs our measurements, since temperature also has a great effect on the electrons in a material. It is therefore often not possible to see the effect of a magnetic field on electrons at room temperature. Therefore, we need to cool our materials as close to absolute zero as possible, -273.15 degrees Celsius. We do this with liquid helium, which is 4 degrees above absolute zero. By playing with pressure differences of the helium and using the isotope helium-3, we manage to cool down our measurements to 0.05 degrees above absolute zero. At this low temperature, almost all temperature interference has been filtered out and we can see new effects in the materials we are researching. With this research we hope to better understand how effects such as superconductivity, quantum fluctuations and magnetism work”.

Sources
(1) Periodiek systeem - informatie over alle elementen - VNCI. Periodiek Systeem. Retrieved September 8, 2020, from https://periodieksysteem.com/element/helium
(2) Wikipedia contributors. (2020, August 7). Helium. Wikipedia. https://nl.wikipedia.org/wiki/Helium
Lithium is the lightest solid element and the metal can even be cut with a knife. However, chances are slim you will ever do this as it does not appear as a metal in nature. In its free state, it is highly reactive and flammable. Usually, it is found in oceans, mineral compounds, and brine. The latter is the main resource in the large-scale industrial production of lithium. This production skyrocketed during the Cold War because lithium is a useful resource for nuclear weapons(1). Nowadays, its purposes are more wholesome. Lithium is often used in heat-resistant ceramics and for lithium-ion batteries. In fact, the 2019 Nobel Prize in Chemistry was awarded to three scientists who developed lithium-ion batteries, which have revolutionized portable electronics(3). Although these batteries are expensive, they can last for decades. This makes them a popular power supply in pacemakers and watches. Also, lithium salts are known mood stabilizers: they can offer a valuable treatment of bipolar disorders(2).
Researchers at Radboud University are studying Lithium-ion batteries. Prof. Arno Kentgens at the Magnetic Resonance Research Center: "Lithium-ion batteries are currently used in most portable devices, such as mobile phones and laptops, because of their high energy per unit mass, compared to other electric energy storage methods. They also have a high power to weight ratio, a high energy conversion efficiency, perform well under high temperatures and have little self-discharge. This is why the current generation of electric cars also has lithium-ion batteries. However, these still have some shortcomings: the weight and price of an electric car is based for over 30% by the batteries, and there are safety risks."
"For a battery with a high energy density, it would be preferred to use a lithium-metal anode, but there is a problem: so-called dendrites will grow between the anode and the cathode, cause the battery to short-circuit. These dendrites easily frow through the separator between the electrodes, which consists of a fluid electrolyte. The fluid electrolytes are highly inflammable and a short circuit could even lead to exploding batteries. This had led to worldwide research on solid electrolytes, which should let lithium-ions pass through easily but do not conduct electrons. Especially the surfaces between the cathode, anode, and the solid play a crucial role. The anode, electrolyte and cathode must react to each other, which is why a protective film is needed, called the ‘solid electrolyte interphase’. It has to be extremely stable and conduct lithium-ions well. NMR is especially a good method to study in detail how lithium moves through the different battery materials and how the structure of a material influences the conductivity of lithium. This is why researchers of Radboud do a lot of research on this, together with colleagues from the Netherlands and abroad."
Sources:
(1) Wikipedia contributors. (n.d.). Lithium. Wikipedia. Retrieved July 24, 2020, https://en.wikipedia.org/wiki/Lithium
(2) Wikipedia contributors. (n.d.). lithium | Definition, Properties, Use, & Facts. Encyclopedia Britannica. Retrieved July 24, 2020, from https://www.britannica.com/science/lithium-chemical-element
(3) Sheikh, K. (2019, October 10). Lithium-Ion Batteries Work Earns Nobel Prize in Chemistry for 3 Scientists. New York Times. https://www.nytimes.com/2019/10/09/science/nobel-prize-chemistry.html?searchResultPosition=4
Beryllium and the corresponding salts have a sweet taste, as was discovered by old-style chemists who thought tasting chemicals was a worthwhile research method. This taste led to the element’s former name Glucine, based on the Greek ‘γλυκύς’ (sweet) that is also found in the word ‘glucose’. Nowadays, it is known that beryllium is highly toxic – and chemists are no longer prone to eat their substances(1). Being exposed to beryllium, for example via dust in the air, can cause a chronic and life-threatening disease called berylliosis. Hence, elaborate control equipment is crucial in any application of beryllium. This was overlooked during World War II, when the demand for fluorescent lights containing beryllium alloys rose. A survey later found that 5 percent of the workers in such manufacturing plants had lung diseases related to beryllium(2). Despite its toxicity, beryllium still has multiple applications due to its unique qualities. For instance, it is one of the lightest metals and it has one of the highest melting points among the light metals, namely an astonishing 1287 degrees Celsius(3). Its lightness and stability over a wide temperature range make it popular in high-speed aircraft, guided missiles, and satellites, including the James Webb telescope. Also, beryllium played a central role in an experiment carried out in 1932 by James Chadwick, which would earn him the Nobel Prize in Physics. He bombarded a sample of beryllium with radium alpha rays and consequently discovered the neutron(2)!
Sources
(1) Beryllium Element Facts. (2012, October 9). Chemicool. https://www.chemicool.com/elements/beryllium.html
(2) Wikipedia contributors. (2020, July 25). Beryllium. Wikipedia. https://en.wikipedia.org/wiki/Beryllium
(3) Live Science. (2017, October 7). Facts About Beryllium. https://www.livescience.com/28641-beryllium.html
Compounds of boron, specifically borax, have been known since ancient times. Boron is produced entirely by cosmic ray spallation – formation of chemical elements from the impact of cosmic rays(1) – and supernovae and is thus a low-abundance element in the solar system and Earth’s crust. Boron was recognized as an element in 1808, when Louis-Josef Gay-Lussac and Louis-Jacques Thénard working in Paris, and Sir Humphry Davy in London, independently extracted boron(3).
Traces of boron are needed for the growth of many land plants and thus indirectly essential for animal life(2). When soil is abundant in boron, plant species can exhibit ‘gigantism’, as boron is important for the growing tips of plant shoots. Boron compounds are being researched and applied in a wide spectrum of biomedical applications. Some are studied as a potential treatment for tumours in the body, so-called boron neutron capture therapy. Upon injection, boron compounds will accumulate in the tumour. Every time a boron nucleus captures a neutron, transmitted by radiation therapy, a tissue-damaging alpha particle is released, which destroys the tumour tissue without affecting the healthy tissue around it(2). The expulsion of such a high energy alpha particle, makes boron isotopes also useful in the nuclear industry, as neutron shields or control rods in nuclear reactors(3).
Sources
(1) Wikipedia contributors. (2020, June 7). Cosmic ray spallation. Wikipedia. Retrieved July 16, 2020, from https://en.wikipedia.org/wiki/Cosmic_ray_spallation
(2) boron | Properties, Uses, & Facts. (n.d.). Encyclopedia Britannica. Retrieved July 16, 2020, from https://www.britannica.com/science/boron-chemical-element
(3) Boron - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of Chemistry. Retrieved July 16, 2020, from https://www.rsc.org/periodic-table/element/5/boron
Without carbon, there would be no life – or at least; not as we know it. Carbon is one of the few elements known since prehistoric antiquity. Carbon serves as a common element of all known life, because it is so abundant, diverse, and able to form polymers at temperatures commonly encountered on Earth(1). The atoms of carbon can bond together in various ways, resulting in different allotropes of carbon. The best known allotropes are graphite, diamond and ‘buckyball’. This last one refers to Buckminsterfullerene; a fused-ring structure resembling a soccer ball (image), made up of molecules of 60 (or 70) carbon atoms. The molecule has been detected in deep space in the interstellar medium spaces between the stars(3).
One recent discovery within the carbon-realm is graphene; an elusive two-dimensional form of carbon(2). Graphene is more than 200 times stronger than steel, an excellent thermal and electrical conductor, flexible, very thin and transparent; which makes it potentially suitable for a wide range of applications. Graphene was discovered by researches in the High Field Magnet Laboratory (HFML) at Radboud University. The 2010 Noble Prize in Physics was awarded to Andre Geim and Konstantin Novoselov, both professors at our Faculty of Science.
As the common element of organic molecules in all known life, carbon is one of the most studied elements in chemistry, as chemical reactions are largely aimed at making or breaking C-C bonds.
Sources
(1) Wikipedia contributors. (n.d.). Carbon. Wikipedia. Retrieved July 13, 2020, from https://en.wikipedia.org/wiki/Carbon
(2) Meer, B. (March 19, 2018). Bekijk: Grafeen. NEMOKennislink. https://www.nemokennislink.nl/publicaties/grafeen/
(3) Wikipedia contributors. (August 20, 2020). Buckminsterfullerene. Wikipedia. Retrieved August 20, 2020, from https://en.wikipedia.org/wiki/Buckminsterfullerene
The fixation of inorganic carbon (CO2) in organic carbon (carbohydrates, proteins, fats) starts with the primary producers (including plants and algae) who convert CO¬2 into glucose (C6H12O6) with the help of light and water. Other species eat the primary producers, creating a food web in which organic carbon is transferred from one organism to another. When organic carbon is used as an energy source, the stored carbon is released again with the help of oxygen in the form of CO2, which completes the circle.
In some ecosystems, such as in bogs, the organic carbon is not completely broken down due to a lack of oxygen in the soil. Here the organic material accumulates in several meters thick packages. When this is covered by sediment and stored in the soil for millions of years, it creates petroleum, natural gas, coal and lignite, or the fossil fuels that we have been using intensively since the 18th century. When it is combusted, the greenhouse gas CO2 is released into the atmosphere, which was no longer present in our biosphere for millions of years, causing an imbalance and warming up our climate. Current peat areas where a lot of carbon is stored have also been drained en masse, releasing the captured CO2 and contributing to global warming.
The big scientific question that this poses for ecologists is: How can we restore ecosystems such as wetlands and bogs so that the balance in biodiversity and carbon exchange is improved? Another question is what the impact of the changing climate is on ecosystems and how sensitive systems are to changes.
Nitrogen was discovered by Daniel Rutherford in 1772. He called it noxious air, because it can extinguish a flame(1). Nitrogen is a common element in the universe. At standard temperature and pressure, two atoms of the element bind to form dinitrogen (N2). Dinitrogen forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen is extremely important to (the origin of) life, as it is an essential component in amino acids, proteins, DNA, and the energy-carrying molecule adenosine triphosphate(1).
Nitrogen is an important component of molecules in every major drug class in pharmacology and medicine, from antibiotics to neurotransmitters and beyond. One important aspect of nitrogen is that it is the only non-metal that can maintain a positive charge at physiological pH(3).
Nitrogen has been in the news a lot in recent years in the "nitrogen crisis". Because the amount of reactive nitrogen on Earth (due to agriculture, fossil fuels, traffic, shipping, etc.) has doubled in the past century, we now have a nitrogen surplus(2). Since nitrogen normally occurs in scarcity, many plants and animals have learned to use it very efficiently and are now overconsuming this previously valuable substance. The plants that grow the fastest, such as nettles, grasses and brambles, overgrow slow growers, which affects insects and birds. In addition, nitrogen acidifies the soil, causing important substances such as calcium to wash away from the soil. The negative effects of a nitrogen surplus are not only visible on land: via rivers, it flushes to the sea, where the nutrient-rich substance causes excessive algae growth that suffocates fish off our coast. It then evaporates and ends up in the air as nitrous oxide, a gas that is an enormous contributor to global warming.
Evan Spruijt, assistant professor Physical Organic Chemistry at Radboud University, tells you more about Nitrogen in the video below.
Click on "Read more" for information about Nitrogen in research at Radboud University.
Microbiology researchers at Radboud University are working with anammox bacteria; essential bacteria for nature and industry, because they can convert noxious nitrogen (in the form of ammonium NH4+) into a harmless variant, namely nitrogen gas (N2). The research on anammox is one of the main projects of the RU Microbiology group and was recently awarded an ERC Advanced grant. With the discovery of anammox bacteria, a missing link was found in the nitrogen cycle. Because of this favourable conversion from which the bacteria get their energy, anammox bacteria are already used in wastewater treatment.
Sources
(1) Wikipedia contributors. (2001, March 23). Nitrogen. Wikipedia. Retrieved August 1, 2020, from https://en.wikipedia.org/wiki/Nitrogen
(2) van Dongen, A., & Voermans, T. (2019, 21 september). Waarom liggen bouwprojecten stil? En 13 andere vragen over stikstof. AD. Retrieved August 1, 2020, from https://www.ad.nl/binnenland/waarom-liggen-bouwprojecten-stil-en-13-andere-vragen-over-stikstof~ad4491d5/
(3) Nitrogen and Phosphorus | Boundless Chemistry. (n.d.). Lumen Learning. Retrieved September 1, 2020, from https://courses.lumenlearning.com/boundless-chemistry/chapter/nitrogen-and-phosphorus/
Oxygen was discovered in 1771 by the Swedish pharmacist Karl Wilhelm Scheele. Later, experiments of the British chemist Joseph Priestley made the gas more widely known(1). It was soon understood that this gas, although only making up one-fifth of the planet's air, allows combustion, as well as the breathing of humans and animals. Oxygen gas is indispensable for many organisms on Earth; without it, there would be no aerobic dissimilation – combustion of organic molecules to generate energy – possible. Oxygen atoms are important components of carbohydrates, which provide energy to living organisms.
In recent years there have been concerns about oxygen levels in the sea, which is declining at an unprecedented rate. Due to global warming and intensive agriculture, oxygen is slowly disappearing from the sea(2). In the past fifty years, the number of so-called ‘dead zones’, where there is no oxygen in the water, has quadrupled. In the dead zones, the oxygen level is so low that most marine animals cannot survive. Fish avoid the spots and have to retreat to smaller habitats.

Sources
(1) Wikipedia contributors. (n.d.). lithium | Definition, Properties, Use, & Facts. Encyclopedia Britannica. Retrieved July 24, 2020, from https://www.britannica.com/science/lithium-chemical-element
(2) Wetenschappers slaan alarm op klimaattop Madrid: ‘Zuurstof in zee neemt razendsnel af’. (2020, December 7). AD. Retrieved July 24, 2020, from https://www.ad.nl/buitenland/wetenschappers-slaan-alarm-op-klimaattop-madrid-zuurstof-in-zee-neemt-razendsnel-af~a3ef133f/
Fluorine is a pale, yellow gas at room temperature. Since the electron configuration of fluorine lacks only one electron to form the stable noble gas neon, the fluorine atom attracts electrons very strongly. Fluorine is therefore by far the most aggressive oxidiser known among the elements and affects almost everything; even materials such as glass or asbestos burn in fluorine gas at room temperature. Because fluorine as a pure gas is such a reactive element, it does not exist naturally in its pure form(1). Salts of fluorine (fluorides) can be found in the Earth's crust, in rocks, coal and clay. Fluorides are released into the air by means of wind blown soil. Small amounts of fluorine naturally occur in water, air, plants and animals(2). Because of this, people come into contact with fluorine through food, drinking water and breathing air. Fluorine can be found in relatively small amounts in all types of food. It is essential for maintaining the strength of the bone system and preventing tooth decay, which is why it is added to toothpaste.
Sources
(1) Fluor. (2012, September 22). Visionair. Retrieved September 8, 2020, from https://www.visionair.nl/wetenschap/het-periodiek-systeem-fluor-f/
(2) Fluor (F). (n.d.). Lenntech. Retrieved September 8, 2020, from https://www.lenntech.nl/periodiek/elementen/f.htm
Neon is a very common element in the universe and solar system (it is fifth in cosmic abundance after hydrogen, helium, oxygen and carbon), but it is very rare on Earth. The reason for this is that neon vaporises quickly and thus forms no compounds to fix it to solids. As a result, it easily escapes the Earth’s atmosphere under the warmth of the sun(2). Neon was discovered in 1898 by the British chemists Sir William Ramsay and Morris W. Travers in London(1). They immediately noted the characteristic red-orange color emitted under spectroscopic discharge. Neon’s distinct glow is still used abundantly in (advertising) lighting(2). Neon is also often used as a heat transport medium in cooling installations.
Neon played an important role in research into the origins of our planet. Previously, researchers were unsure whether the planet formed relatively quickly from a cloud of gas and dust around the sun, or if it took long time, with gases and water brought in later by meteorites. Research into neon in the Earth’s mantle has shown that it must have been the first scenario; the Earth was formed relatively quickly, within a few million years, from the cloud of gas and dust in which the young sun was then still enveloped(3).
Click on the button below to read about neon's use in research at the FELIX Laboratory at Radboud University.
Sources
(1) Neon (Ne). (n.d.). Lenntech. Retrieved July 15, 2020, from https://www.lenntech.nl/periodiek/elementen/ne.htm
(2) Wikipedia contributors. (2020, April 16). Neon. Wikipedia. Retrieved July 15, 2020, from https://en.wikipedia.org/wiki/Neon
(3) E.E. (n.d.). Neon uit aardmantel wijst op snelle vorming van de aarde. Zenit. Retrieved July 15, 2020, from https://zenitonline.nl/neon-uit-aardmantel-wijst-op-snelle-vorming-van-de-aarde/
Neon is used in research at Radboud University at the FELIX Laboratory (Free Electron Lasers for Infrared Experiments). “In our lab, we are mainly interested in the structure and reactivity of molecular ions.”, says Daniel Rap, PhD student at FELIX. “Infrared spectroscopy can be used to study the vibrations of molecules in order to determine the 3D structure of the molecule. However, when studying ions, the traditional way of infrared spectroscopy (that is, measuring absorption of the irradiated light) does not work because we are talking about a few (ten) thousand ions that we want to study. There is then too little difference between the incoming and outgoing light to measure. This is where neon comes into play. Under cold conditions (6 Kelvin; -267 °C), we can connect the molecular ion and the neon atom with a very weak bond. In this way, neon functions as a 'tag', as it were: when we use the infrared laser, the energy of the light causes the neon atom, which is very weakly bound, to become detached from the ion. In this way we can count how many of the ion / neon complexes we have at which wavelength of the infrared light. And this gives us the possibility to measure an infrared spectrum of a few thousand ion / neon complexes and thus determine the structure of the ion.”
Sodium is an essential element for life. As part of salts, it is found in almost any biological material(1). Its importance in your own body should not be taken with a grain of salt either. For example, it regulates blood pressure and osmotic processes. For a less abstract image, picture that sodium is one of two building blocks in common salt (NaCl). The element’s name comes from the Latin word ‘sodanum’. This was a compound containing sodium, used for headache relieve in Medieval Europe. In the present day, sodium is used to produce soap, glass, paper, textiles, synthetic rubber, pharmaceuticals, and so on. In other words, it is of immense commercial importance. However, do not think that sodium metal is easy to handle. It is extremely reactive – generally more so than lithium – and hence does not occur freely in nature. In fact, pure sodium powder may explode when it touches even the slightest amount of water, like the moisture on your hand(2).
Sources:
(1) sodium | Facts, Uses, & Properties. (n.d.). Encyclopedia Britannica. Retrieved July 27, 2020, from https://www.britannica.com/science/sodium
(2) Wikipedia contributors. (n.d.). Sodium. Wikipedia. Retrieved July 24, 2020, from https://en.wikipedia.org/wiki/Sodium
Without magnesium, life as we know it would not exist. It lies at the core of chlorophyll, which in turn lies at the core of photosynthesis in plants, which in turn lies at the core of the food chain. Hence it is essential to all plant and animal life, including your own body, where it is indispensable for hundreds of enzymes. For example, “your nervous system could not function without it, because it is important for nerve impulse conduction”, explains Sharon Kolk, associate professor in Neurobiology at Radboud University. “It also supports memory and learning.” Luckily, deficiencies are rare because human skeletons carry sufficient storage. Otherwise you could eat some magnesium-rich food like almonds, soybeans, cashew nuts and chocolate(1). However, magnesium also has a history of not creating but destroying life. Although it is hard to ignite in bulk, its fires are very hard to extinguish as water only stimulates the combustion. This property was exploited during firebombing of cities during World War II, where civilians could only try to smother fire with dry sand(2). The brilliant light it produces when ignited is also used for more friendly applications like photography, flares and pyrotechnics(3). It is also used when products can benefit from being lightweight, such as laptops and race bikes (a steel frame can be nearly five times as heavy as a magnesium one). People have also been using magnesium compounds as laxatives for centuries(1).
Sources
(1) Magnesium - Element information, properties and uses | Periodic Table. (n.d.). www.rsc.org. Retrieved August 3, 2020, from https://www.rsc.org/periodic-table/element/12/magnesium
(2) Wikipedia contributors. (n.d.). Magnesium. Wikipedia. Retrieved July 24, 2020, from https://en.wikipedia.org/wiki/Magnesium
(3) Magnesium Element Facts. (2012, September 13). www.chemicool.com. Retrieved July 24, 2020, from https://www.chemicool.com/elements/magnesium.html
Compounds of aluminium have been known since ancient times. Only in the beginning of the 19th century researchers managed to extract aluminium in its pure form. For a long time after this, aluminium was so valuable that it was mainly used in decorative or luxurious ornaments. In pure form aluminium is soft and not strong. But in alloys with small amounts of copper, magnesium, silicon, manganese or other metals, it is suitable for all kinds of applications. Aluminium is the most common metal on Earth, but it does not exist in its pure form. It takes a lot of energy to isolate it from ore (bauxite). For a long time it was more expensive than gold, until technology was developed in the 19th century for large-scale aluminium production(1). The metal has been available for a little over a century now, and during that time it has stormed the world. Aluminium is light, durable and resistant to corrosion. It is a good conductor, it does not spark, and it forms relatively easily(2). Aluminium is therefore not only applicable in many consumer products - such as packaging material, toys, household equipment (pans, cutlery), camping equipment (folding chair, tent poles), furniture or even food colouring - it also plays an important role in more industrial applications, such as the automotive industry, power lines, lightning rods, antennas, and the light and sound industry.
Sources
(1) Periodiek systeem - informatie over alle elementen - VNCI. (z.d.). Periodieksysteem. Retrieved July 15, 2020, from https://periodieksysteem.com/element/aluminium
(2) Wikipedia contributors. (n.d.). Aluminium. Wikipedia. Retrieved July 15, 2020, from https://nl.wikipedia.org/wiki/Aluminium
Silicon makes up 27.7% of the Earth’s crust by mass and is the second most abundant element on the planet, after oxygen. Silicon is one of the most useful elements to mankind(1): it is the principal component of glass, cement, concrete, steel, bricks, most semiconductor devices, and silicones(2). The element silicon is used extensively as a semiconductor in solid-state devices in the computer and microelectronics industries. That is why the high-tech region of Silicon Valley in California is named after silicon(3), and the late 20th century to early 21st century period has even been described as the Silicon Age, due to the element’s large impact on the modern world economy.
Sources
(1) Silicon - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of Chemistry. Retrieved July 16, 2020, from https://www.rsc.org/periodic-table/element/14/silicon
(2) Silicon (Si) - Chemical properties, Health and Environmental effects. (z.d.). Lenntech. Retrieved July 20, 2020, from https://www.lenntech.com/periodic/elements/si.htm
(3) Wikipedia contributors. (n.d.). Silicium. Wikipedia. Retrieved July 20, 2020, from https://nl.wikipedia.org/wiki/Silicium
Phosphorus was discovered in 1669 after an unusually filthy experiment: Hennig Brand, a German physician and alchemist, boiled, filtered and processed as many as 60 buckets of urine to isolate the element phosphorus(1). Brand called the substance he had discovered ‘cold fire’ because it was luminous, glowing in the dark(2). Phosphorus has three main allotropes: white, red, and black.
One of the most abundant usages of phosphorus is as a fertiliser: in the process of converting phosphate rock to usable materials, calcium hydrogen phosphates are formed, which are important to make fertiliser or food supplements for animals(3). Besides its use in fertilisers, elemental phosphorus applications include fireworks and matches, because it allows combustion at the temperature of frictional heat(4).
Concerns have arisen about phosphorus use, however. On its journey from mining to consuming (by animals, but also indirectly; humans), most of the phosphorus is wasted and ends up in waterways where it can cause algal blooms.
Phosphorus (P) is an essential element in biology, and mainly occurs in the environment as PO4. This is an essential nutrient for organisms and an important component of DNA, RNA, ATP and phospholipids.
The ratio of phosphorus compared to other elements (like carbon and nitrogen) in organisms is a property that is used to indicate the degree of which an organism is a nutritional resource for grazing animals and predators.
Many ecosystems, such as lakes and grasslands, have limited phosphorus. An increase of phosphorus, for example via a fertiliser, can lead to an explosive growth of plants in the ecosystem. The department of Aquatic Ecology & Environmental Biology conducts research on the ecological restoration of ecosystems that have a lot of phosphorus due to fertilisation in the past. This can be done by digging up the soil with a lot of phosphorus or by growing plants that easily take up phosphorus from the environment, such as Azolla.
In lakes, an abundance of phosphorus can make a clear ecosystem with submerged plants turn into a troubled state with (poisonous) algae. Next to the shift in species, this turn can influence other ecosystemic processes, such as methane production in the sediment.
Sources
(1) Gagnon, S. (n.d.). It’s Elemental - The Element Phosphorus. JLab Science Education. Retrieved July 16, 2020, from https://education.jlab.org/itselemental/ele015.html
(2) Stewart, D. (2012, September 28). Phosphorus Element Facts. Chemicool. https://www.chemicool.com/elements/phosphorus.html
(3) Gregersen, E. (z.d.). phosphorus | Definition, Uses, & Facts. Encyclopedia Britannica. Retrieved July 16, 2020, from https://www.britannica.com/science/phosphorus-chemical-element
(4) Cunningham, J. M. (n.d.). Match. Encyclopedia Britannica. Retrieved July 16, 2020, from https://www.britannica.com/science/match-tinder#ref15123
Sulfur is the fifth most common element on Earth and has been known and used since ancient times. It is mentioned – sometimes referred to as brimstone, ‘burning stone’ – in ancient India, Greece, China and Egypt. Elemental sulfur is mostly found near hot springs and volcanic regions, perhaps explaining why in the Bible sulfur or brimstone is often associated with hell and fury(1).
The majority of the sulfur produced today comes from underground deposits through a process known as the Frasch process(2). Today, its most common use is in the manufacture of sulfuric acid, which is used in fertilisers, batteries and cleaners. It is also used to refine oil and in processing ores(1). Many sulfur compounds are odorous and responsible for, among others, the smell of skunk, grapefruit, garlic, rotting eggs and other biological processes.
Volatile sulfur compounds are very malodorous and toxic, and are also produced in a number of industrial processes. Furthermore, they have a major impact on global warming and acid precipitation processes. Microbiology researchers at Radboud University are investigating the microbial degradation of volatile organic sulfur compounds. Studies of the degradation are coupled to the application of promising bacterial isolates in treatment of polluted air.
You can read more about this research at: ru.nl/microbiology/research
Sources
(1) Pappas, S. (2017b, September 29). Facts About Sulfur. Live Science. Retrieved July 16, 2020, from https://www.livescience.com/28939-sulfur.html
(2) Gagnon, S. (n.d.). It’s Elemental - The Element Sulfur. JLab Science Education. Retrieved July 16, 2020, from https://education.jlab.org/itselemental/ele016.html
At room temperature, chlorine is a yellow-green gas. It is an extremely reactive element and a strong oxidiser: among the elements, it has one of the highest electron affinities and the third-highest electronegativity. Chlorine is too reactive to occur in its native form in nature but is very abundant in the form of chloride salts. The most common chloride salt is sodium chloride (table salt) and has been known since ancient times. Archaeologists have found evidence that rock salt was used as early as 3000 BC and brine as early as 6000 BC(1).
The high oxidising potential of elemental chlorine led to the development of commercial bleaches and disinfectants. It is the most commonly used drinking water disinfectant in the world and protects millions of people against pathogenic microbes. It is also used as a reagent for many processes in the chemical industry.
Sources
(1) Weller, O., & Dumitroaia, G. (2005, December). Antiquity, Project Gallery: Weller & Dumitroaia. Antiquity. Retrieved July 16, 2020, from https://web.archive.org/web/20110430145935/http://antiquity.ac.uk/ProjGall/weller/
The name argon is derived from the Greek ἀργος, which means ‘lazy’ or ‘non-active’. A fitting name, considering that argon is colourless, odourless, non-flammable and nontoxic as a solid, liquid or gas. It is chemically inert under most conditions and does not form stable compounds at room temperature(1). Since the 18th century, chemists predicted that there would be a gas like argon in the air. But it was not until the late 19th century that Lord Rayleigh and William Ramsay experimentally proved that argon actually existed. The Earth’s atmosphere consists of 0,94% argon. It is now produced as an industrial gas; for example as filler in light bulbs – argon prevents the filament from burning at high temperatures – or as blue light- because like neon, it emits a bright colour under spectroscopic discharge. Because of its inert characteristic, it is also used as protection in processes like welding, titanium production, or the development of crystals(2).
Sources
(1) Wikipedia contributors. (2020d, July 9). Argon. Wikipedia. Retrieved July 21, 2020, from https://en.wikipedia.org/wiki/Argon
(2) Lenntech. (n.d.). Argon (Ar). Lenntech. Retrieved July 21, 2020, from https://www.lenntech.nl/periodiek/elementen/ar.htm
The name potassium is derived of the Dutch word “potas” (pot ash), because potassium carbonate was originally obtained by leaching wood and then heating the substance to 'ash' in a pot(1). Ironically, in Dutch and other Germanic languages the word for potassium (‘kalium’) comes from the Arabic “al-qalyah” (meaning ‘ash from plants’), whereas almost all other languages use the Dutch derivative. In the 19th century, it was discovered that potassium salts are also extractable from mines. This put an end to virtually all extraction of potash from wood in Western Europe and North America(3).
As an alkali metal, potassium has a single outer shell electron, that is easily removed to create an ion with a positive charge (cation). Because of this positive charge, the potassium ion easily combines with ions that have a negative charge (anions) forming a salt. Therefore, potassium in nature occurs only in salts(2). Potassium ions are vital for the functioning of all living cells; it is necessary for normal nerve transmission. Fresh fruits and vegetables are good dietary sources of potassium.
Potassium has been used at Radboud University in research by the Scanning Probe Microscopy department (SPM). The scanning tunneling microscope is a measuring instrument at the Institute for Molecules and Materials, used to examine fundamental physics on the atomic scale.
Click on the button "Read more" to find out more about this research at Radboud University.
Sources
(1) Wikipedia contributors. (2020a, February 16). Kalium. Wikipedia. Retrieved July 20, 2020, from https://nl.wikipedia.org/wiki/Kalium
(2) Wikipedia contributors. (2020j, July 24). Potassium. Wikipedia. Retrieved July 20, 2020, from https://en.wikipedia.org/wiki/Potassium
(3) NutriNorm (2020, July 27) - Waar komt kalium vandaan. (n.d.). Nutrinorm. Retrieved July 20, 2020, from https://www.nutrinorm.nl/nl-nl/Paginas/Hoofdelementen-Waar-komt-kalium-vandaan.aspx#.Xx6ipkUzZPY
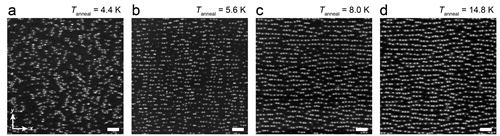
Potassium is an alkali metal and thus has one outer shell electron that is easily donated to its surroundings. This quality is useful in experiments involving doping. Research conducted at Radboud University concerned the structural arrangements of potassium atoms on the surface of black phosphorus. “The experiment was actually initiated accidentally”, Elze Knol, PhD student at Scanning Probe Microscopy, explains. “We were preparing a doping experiment with potassium atoms on black phosphorus, which is supposed to be kept under very cold circumstances (T < 5 K). One time, we forgot to add liquid helium to the STM, to keep the temperature down. The temperature started creeping up and after cooling back down, we noticed that the potassium atoms had moved and were now forming organized rows on the black phosphorus. That triggered our curiosity; what is happening here?”. In the experiment that followed, it was found that the potassium atoms were not distributed equally in all directions, but in the x-direction the atoms were much closer to each other than in the y-direction. This indicates that the black phosphorus screens the donated electrons from the potassium atoms in an anisotropic manner – unlike isotropic materials, which have identical physical properties in all directions.
Let’s put calcium in the limelight, literally. Lime is calcium oxide, which can be burnt with a special flame to create an intense light. This technique was used in the 19th century to light theatre stages, hence the expression. Nowadays we have light bulbs, but calcium still serves other purposes. For example, both humans and snails use it to build their houses, although only humans use it in cement. This building technique is age-old: the Romans used it in constructing aqueducts and amphitheatres(1) and lime was already used in buildings about 9000 years ago. It is no surprise that the element’s name is based on ‘calx’, the Latin word for lime. Of course, calcium also has many crucial biological functions. Sharon Kolk, associate professor in Neurobiology at Radboud University: “It is the most abundant metal in the human body and important in electrical signalling within the nervous system. It plays a key role in the release of neurotransmitters by neurons in the brain. This is very important for memory storage and retrieval. It is furthermore key to the maintenance of our blood pressure.” An average human body contains roughly 1 kg of calcium, of which 99% is stored in the bones. It can enter your body via foods like spinach, milk and almonds(2).
Sources
(1) Calcium Element Facts. (2012, October 4). Chemicool. Retrieved August 6, 2020, from https://www.chemicool.com/elements/calcium.html
(2) Calcium - Element information, properties and uses | Periodic Table. (z.d.). www.rsc.org. Retrieved August 6, 2020, from https://www.rsc.org/periodic-table/element/20/calcium
Dmitri Mendeleyev already knew when he created his periodic table that there was an element missing that would be between calcium and titanium. He referred to it as ‘eka-boron’ because the element would be placed under boron in his table. Ten years later, in 1879, Swedish chemists stumbled upon the element, which was then named scandium because of its Scandinavian discoverers(1). Scandium is mostly used for research purposes(2). Global-scale, industrialized mining and production of scandium only really started in the 1970s, when its positive effects on aluminium alloys were discovered. This is still the most used application of scandium; used in LCD televisions, mobile phones, racing and mountain bikes. Scandium is almost as light as aluminium but it has a much higher melting point, which is now being researched for its usefulness in space exploration.
Sources
(1) Wikipedia contributors. (2018, November 25). Scandium. Wikipedia. Retrieved August 6, 2020, from https://nl.wikipedia.org/wiki/Scandium
(2) Scandium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of Chemistry. Retrieved August 6, 2020, from https://www.rsc.org/periodic-table/element/21/Scandium
Titanium is found abundantly on Earth, but due to its reactive nature, it is mostly bound to other elements in minerals and soils. It is also present in plants, animals and natural waters(1). Some meteorites and stars contain high concentrations of titanium. For example, rocks that Apollo 17 brought back from the Moon, consisted of 12.1% titanium dioxide(2). As a lightweight, high-strength, low-corrosion metal, titanium is used in alloy form for components in aircrafts, space crafts, missiles and ships. It also is used in prosthetic devices, because it does not react with fleshy tissue and bone(1).
“Titanium is an element used in a wide range of applications, either as a pure element or in specialized alloys. Being an excellent getter material, we use it to trap gas molecules that sporadic hit titanium covered chamber walls to maintain an ultra-high vacuum. Additionally, due to its two unpaired electrons in the atomic shell, it can exhibit interesting magnetic properties”, writes Manuel Steinbrecher, post-doc at Scanning Probe Microscopy.
“Within the experiments in our group, we have deposited single Ti atoms on an insulating magnesium oxide (MgO) surface. However, due to its aforementioned exceptional getter capabilities, we mostly observe the hydrogenated TiH molecule on the surface. Interestingly, TiH is one of the most abundant elements in outer space, but experimental data to confirm its electronic structure is still lacking. Using a ‘’scanning tunneling microscope’’ (STM) at ultra-low temperatures allows us to address and study individual TiH molecules. By irradiation with radiofrequency, we perform electron spin resonance (ESR) experiments, that relies on the same principle of magnetic resonance that MRI scans use for medical diagnostic With this technique and the help of cutting-edge quantum chemistry calculations, we have, e.g., studied the molecule’s conformational and electronic structure as well as its magnetic properties with a precision as never before. In the future, the ESR technique will enable us to examine different kinds of sample systems with a precision the standard STM technique cannot provide.”
Sources
(1) Augustyn, A. (n.d.). titanium | Properties, Uses, & Facts. Encyclopedia Britannica. Retrieved July 21, 2020, from https://www.britannica.com/science/titanium
(2) Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Los Alamos National Laboratory. Retrieved July 21, 2020, from https://periodic.lanl.gov/22.shtml
Vanadium was first discovered in 1801 by the Spanish-Mexican mineralogist Andrés Manuel del Rio. He named it panchromium, after the Greek ‘παγχρώμιο’, meaning 'all colours', because its salts had all kinds of different colours(1). But that name didn’t last long, because a French chemist erroneously convinced Del Rio he had not discovered a new element, but just a impure form of chromium. Thirty years later, Del Rio’s findings were proven right when a Swedish chemist ‘rediscovered’ the element. Since then, it was called vanadium, in honour of the Scandinavian goddess Vanadís, also a testament to its multicoloured compounds(2). Vanadium is a medium-hard, steel-blue metal. It is rare in nature in its native form, but compounds of vanadium are found naturally in minerals, mostly vanadite and carnotite. Most vanadium used today is processed in steel, making it stronger, lighter and stainless(3).
The chemistry of vanadium is noteworthy for its oxidation states. Vanadium can form compounds with water (solution for metal salts) of which the colours are lilac, green, blue and yellow-orange.

Sources
(1) Wikipedia contributors. (2019a, 11 February). Vanadium. Wikipedia. Retrieved July 21, 2020, from https://nl.wikipedia.org/wiki/Vanadium
(2) Mindat. Vanadium: Mineral information, data and localities. (n.d.). Mindat. Retrieved July 21, 2020, from https://www.mindat.org/min-43604.html
(3) Blekemolen, J. (2020, 3 July). De beste vanadium aandelen van dit moment. Online Broker LYNX. Retrieved July 21, 2020, from https://www.lynx.nl/kennis/artikelen/beste-vanadium-aandelen-beleggen/
Chromium is a steely-grey, hard and brittle transition metal. It is the most important additive in stainless steel, mainly for its anti-corrosive properties(1). Chromium is also highly valued as a metal that can be highly polished without the common by-effect of corrosion. The name chromium is derived from the Greek word ‘chróma’, meaning colour, because many chromium compounds are coloured(2): the green colour of emerald, serpentine, chrome mica, and the red colour of ruby are due to small amounts of chromium(3). Chromium is the third hardest element behind carbon and boron. Because of its resistance to corrosive reagents, chromium is extensively used as an electroplated protective coating(3). For example, the compound chromium-6 has often been used in anticorrosion paint, but was recently proved to be harmful to humans. Chromium-6 is released when spraying with chrome-containing paint or by sanding, grinding, sawing or heating the painted surface. Dust that contains chromium-6 is then released into the air, which can cause cancer, among other things.
The element was discovered in 1797, but it would take until 1898 before pure chromium was extracted. By then ores and compounds of chromium were already extensively used, for example in leather tanning and steelmaking(4). Excavated swords and crossbow bolts found in burial pits suggests that chromium coating was even used as early as the third century BC(5).
Sources
(1) Wikipedia contributors. (n.d.). Chromium. Wikipedia. Retrieved July 13, 2020, from https://en.wikipedia.org/wiki/Chromium
(2) Haal de feiten over de Element Chromium. (2019, July 3). Greelane. Retrieved July 13, 2020, from https://www.greelane.com/nl/science-tech-math/wetenschap/chromium-element-facts-606519/
(3) Rafferty, J. P. (n.d.). Chromium | chemical element. Encyclopedia Britannica. Retrieved July 21, 2020, from https://www.britannica.com/science/chromium
(4) Bacon, F. E., & Downing, J. H. (n.d.). Chromium processing. Encyclopedia Britannica. Retrieved July 21, 2020, from https://www.britannica.com/technology/chromium-processing
(5) Cotterell, Maurice. (2004). The Terracotta Warriors: The Secret Codes of the Emperor's Army. Rochester: Bear and Company. ISBN 1-59143-033-X. Page 102.
Manganese is a hard, brittle, silvery metal. It is in fact so brittle, that it isn’t of much use as a pure metal. It is especially useful as an alloy, especially in stainless steels(1). Manganese is often found in minerals in combination with iron. It is also an essential element in all known living organisms, especially in many types of enzymes. For example, the enzyme used during photosynthesis contains atoms of manganese(2). Furthermore, manganese is key to normal cell function and metabolism.
Manganese is the fifth most abundant metal in the Earth’s crust. Its minerals are broadly scattered, pyrolusite (manganese dioxide) and rhodochrosite (manganese carbonate) being the most common. Pre-historic cave painters in France used pyrolusite (a black ore) as early as 30,000 years ago. In more recent times it was used by glass makers to remove the pale greenish tint of natural glass(2).
“Manganese is an essential metal that plays a fundamental role in brain development and functioning. Environmental exposure to it may lead to accumulation in the basal ganglia and development of Parkinson-like disorders”, explains Sharon Kolk, associate professor in Neurobiology and brain development at Radboud University. Recently researchers are focusing on early-life overexposure to manganese and the potential vulnerability of younger individuals to toxicity also in regard to cognitive and executive functions through the involvement of the frontal cortex.
Sources
(1) Wikipedia contributors. (2020g, July 16). Manganese. Wikipedia. Retrieved July 21, 2020, from https://en.wikipedia.org/wiki/Manganese
(2) Manganese - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of Chemistry. Retrieved July 21, 2020, from https://www.rsc.org/periodic-table/element/25/manganese
Iron is the fourth most common element in the Earth's crust. Humans started to master the process of extracting usable metal from iron ores around 2000 BC, although excavations have shown that iron was already being used in old Egypt around 4000 BC, but it took a long time before the use of iron spread and it became (practically and financially) accessible. Copper alloys used in tools and weapons were gradually being replaced by iron. That transition marks the change from the Bronze Age to the Iron Age, around 1200 BC. Nowadays, iron alloys such as steel and cast iron are by far the most common industrial metals(1). The large amount of iron in the Earth is thought to contribute to its magnetic field(2).
Iron is required for life because it is a key element in the metabolism of all living organisms. It is an essential component of numerous proteins and enzymes that support essential biological functions, such as oxygen transport, energy production, and DNA synthesis(3). “It is also important for cognitive functioning as it plays an important role in myelination, monoamine synthesis and neuron/glia energy metabolism”, explains Sharon Kolk, associate professor in Neurobiology at Radboud University. Iron deficiency in children has been associated with poor cognitive development, poor school achievement, and abnormal behaviour patterns.
Microbiology researchers at Radboud University are investigating anammox bacteria. These bacteria have a red colour because they contain a large amount of cytochromes; proteins with one or more central iron ions (= an electrically charged atom). Cytochromes perform important conversions in the energy metabolism of the anammox bacteria; they can convert noxious nitrogen into harmless nitrogen gas, which is especially important in the current ‘nitrogen crisis’ (read more about this under element 7: Nitrogen).
Click on "Read more" for information about Iron in research at Radboud University.
Sources
(1) Wikipedia contributors. (n.d.). Iron. Wikipedia. Retrieved July 18, 2020, from https://en.wikipedia.org/wiki/Iron
(2) Iron. (2020, June 5). BYJUS. Retrieved July 18, 2020, from https://byjus.com/chemistry/iron/
(3) Hidgon, J., & Wessling-Resnick, M. (2001). Iron. Linus Pauling Institute. https://lpi.oregonstate.edu/mic/minerals/iron
“Iron is the second most abundant element in the Earth’s crust and therefore plays a prominent role in our everyday life. Besides cobalt and nickel, it is one of the three elements, that are ferromagnetic at room temperature. And yet, being widely used in all kind of applications, its magnetic properties are still not fully understood on a fundamental level”, writes Manuel Steinbrecher, post-doc at Scanning Probe Microscopy.
“As a group interested in understanding the transition from microscopic (single atoms) to macroscopic (ensembles of many atoms) magnetism, iron is an appealing element to study. Working in vacuum conditions better than in interstellar space and at temperatures down to -273.1°C (30 milliKelvin above absolute zero temperature) allows us to place single Fe atoms on atomically clean surfaces and study their magnetic properties with astonishing precision. To probe and manipulate a single (iron) atom we use a device called ‘’scanning tunneling microscope’’ (STM). Depending on the substrate of choice (e.g. platinum, copper, magnesium oxide etc), iron’s magnetic properties change due to the interplay of the atom’s with the substrate’s electrons. Furthermore, creating artificial magnetic structures atom-by-atom via atom manipulation allows us to perform experiments in the microscopic as well in the macroscopic regime, on the same platform and with ultimate control. In the future, this will allow a better understanding of fundamental questions in the field of magnetism and might provide opportunities to create atomically precise spintronic devices.”
Cobalt is a brittle, hard, silver-grey transition metal with magnetic properties comparable to those of iron. The element itself was discovered in 1730 by Swedish scientist Georg Brandt. Long before that, cobalt compounds were already used in ancient Egypt, Greece and the Roman Empire to colour glass. A necklace with cobalt-coloured blue glass beads from about 2250 BC was found in Persia. Cobalt is still used as drying agents for paints and inks(2).
Compounds of cobalt are also important as oxidation catalysts in a number of industrial processes(1), for example the cobalt carboxylates (known as cobalt soaps). Cobalt is required in small amounts for life and is the only metal found in vitamins (cobalt is the critical component of vitamin B12).
Scientists in the Scanning Probe Microscopy group (SPM) at Radboud University discovered a new mechanism for information storage in the smallest unit of matter: a single atom of cobalt on the surface of black phosphorus. Brian Kiraly and Werner van Weerdenburg (a post-doc and PhD student in the SPM department) discovered that a single cobalt atom on the black phosphorus surface can be in two different states that differ in their orbital configurations (3). Brian Kiraly: “You can imagine the two states as two wells with an energy barrier in between. The atom is stable in its state, unless enough energy is supplied to cross the barrier.” And that’s exactly what he and his colleagues figured out: to flip the state of the atom (0 or 1) back and forth, in a controlled way. And voilà: one single atom can now store information, which can be read out and rewritten. Does this mean that this new technique can be implemented in our smartphones in the next few years? Well, the current experiments were performed at a very low temperature (-269°C), but it does show promise for operation at higher temperatures. The benefit of using the orbital memory is that the energy barrier is higher, compared to magnetic information storage in a single atom, so storage is more robust. A remaining problem is that at room temperature, the atoms can move around on the surface. “You can imagine that it’s not exactly ideal if the bits that you want to read out for information, keep hopping around.”, Werner van Weerdenburg explains. Nonetheless, this research is an important step to denser and more energy-efficient storage of information. And this will have a tremendous impact, when you consider that our computers are currently demanding more than 5 percent of the world’s electricity.
This orbital memory technique, which allows for information storage at the smallest scale, opens up a world of future possibilities. So of course, the researchers didn’t stop there. Implementing the concept of orbital memory utilizing scanning tunneling microscopy, they are now working on research involving atomic neural networks.
Curious? Then the Master’s programme Physics of Molecules and Materials is your best entry. In Alex Khajetoorians’ course “Scanning Probe Microscopy” discusses recent research projects. There is also a possibility to do a Master’s internship at the Institute for Molecules and Materials, in which you will be deeply involved with ongoing research.
Sources
(1) Cobalt. (z.d.). Web Elements. Geraadpleegd 22 juli 2020, van https://www.webelements.com/cobalt/
(2) Hawkins, M. (2001). "Why we need cobalt". Applied Earth Science. 110 (2):
66-71. doi:10.1179/aes.2001.110.2.66
(3) Kiraly, B., Rudenko, A. N., van Weerdenburg, W. M. J., Wegner, D., Katsnelson, M. I., &
Khajetoorians, A. A. (2018). An orbitally derived single-atom magnetic memory. Nature
Communications, 9(1), 3904. https://doi.org/10.1038/s41467-018-06337-4
Nickel is silvery white, tough and harder than iron. It is generally known because of its use in coinage(1). Research indicated that nickel was already being used around 3500 BC in several bronze items and to give glass a green colour. Nowadays it is mainly used as pure metal or in the form of alloys for many household and industrial applications, for example to produce stainless steel(2). Nickel slowly oxidises by air at room temperature and is corrosion-resistant. Compounds of nickel are also used to catalyse the hydrogenation of unsaturated fats and oils(1).
The name nickel finds it origins in a 17th-century German tale(3). In the 1600s, German miners searching for copper stumbled upon a previously unknown ore, a pale brownish-red rock. They thought they had discovered another copper ore and attempted to extract the copper – to no avail. The frustrated miners blamed Nickel, a malicious demon in German mythology, and began calling the ore ‘kupfernickel’, which translates to ‘copper demon’. About a century later, in 1751, a Swedish alchemist experimenting with the ore, found that various properties clearly revealed that it wasn’t copper. He extracted nickel and isolated it as a new element; he dropped the ‘kupfer’-part and called the new element nickel.
Sources
(1) nickel | Definition, Properties, Symbol, Uses, & Facts. (z.d.). Encyclopedia Britannica. Retrieved July 22, 2020, from https://www.britannica.com/science/nickel-chemical-element
(2) Wikipedia contributors. (2020e, July 12). Nickel. Wikipedia. Retrieved July 22, 2020, from https://en.wikipedia.org/wiki/Nickel
(3) Pedersen, T. (2016, 23 September). Facts About Nickel. Live Science. Retrieved July 22, 2020, from https://www.livescience.com/29327-nickel.html
Copper is, with gold, the oldest known element. It is one of the few elements that can occur in nature in its native metallic form. Researchers discovered copper was already being used 11,000 years ago, then as copper beans, found in northern Iraq. It was the first metal that humans learned to smelt, cast into a shape, and alloy with other metals(1). Copper is key to proper functioning of the immune system (antioxidant activity), blood vessels, nerves (neurotransmitter synthesis, neuronal and glial energy metabolism), and bones.
Olga Luschikova, PhD student Condensed Matter Physics at Radboud University, is studying the role of copper nanoparticles in the production of sustainable fuels. You can learn more about this by watching the video below.
Click on "Read more" to find out about how copper is used in research at Radboud University
Sources
(1) Wikipedia contributors. (n.d.). Copper. Wikipedia. Retrieved August 5, 2020, from https://en.wikipedia.org/wiki/Copper
Copper is also being researched at the Scanning Probe Microscopy department (SPM). The scanning tunnelling microscope is a measuring instrument at the Institute for Molecules and Materials, used to examine fundamental physics on the atomic scale. “We are investigating electrons that travel on the surface of copper (to be precise, the Cu(111) surface)”, writes Wouter Jolie, post-doc at the Scanning Probe Microscopy. “Those can be treated as a nearly-free, two-dimensional electron gas, an ideal starting point for our fundamental research. We were able to visualise these "electronic waves" with our scanning tunnelling microscope, as shown by the famous quantum corral experiment. Our focus right now is to artificially pattern the surface of copper with single atoms or molecules to transform these waves into new exotic phases of matter that have been postulated theoretically, but never found in real materials up to date.”

Zinc is the 24th most abundant element in the Earth’s crust, with the largest (workable) lodes in Australia, Asia and the United States. Brass, an alloy of zinc and copper, was used as early as the 3rd millennium BC. Although evidence shows that zinc production sites date back to the 6th century BC, it wasn’t produced on a large scale until the 12th century, in India (1). Now, the element is mainly used in corrosion-resistant zinc plating of iron. Other applications include batteries or castings, and compounds of zinc can be found in dietary supplements, deodorants, shampoos and paint. Zinc is a micronutrient; a mineral essential to organisms, including humans.
Researchers at Radboudumc have discovered how zinc inhibits the development of macular degeneration (AMD); an age-related eye disease that gets worse over time. Small abnormalities in the genes of the complement system cause a slight over activity of the complement system - an important part of the immune system, which leads to an accelerated wear of the yellow spot (macula) of the eye. Zinc "calms" that system, inhibiting the development of old-age blindness (2). This discovery could have far-reaching consequences for a variety of other conditions, in which the complement system also plays a role.
Sources:
(1) Wikipedia contributors. (n.d.). Zinc. Wikipedia. Retrieved 2 February 2021, from
https://en.wikipedia.org/wiki/Zinc
(2) Smailhodzic, D., van Asten, F., Blom, A. M., Mohlin, F. C., den Hollander, A. I., van de Ven, J. P. H.,
van Huet, R. A. C., Groenewoud, J. M. M., Tian, Y., Berendschot, T. T. J. M., Lechanteur, Y.
T.E., Fauser, S., de Bruijn, C., Daha, M. R., van der Wilt, G. J., Hoyng, C. B., & Klevering, B. J.
(2014). Zinc Supplementation Inhibits Complement Activation in Age-Related Macular
Degeneration. PLoS ONE, 9(11), e112682. https://doi.org/10.1371/journal.pone.0112682
Gallium is a soft, silver-white metal that is a brittle solid at a lower temperature. Gallium does not occur as a free element in nature, but compounds of gallium are found in zinc ores and bauxite. Because gallium is a soft metal which melts quite easily (it will melt in your hand due to your body temperature), gallium is mainly used as an alloying element to make alloys that melt at low temperatures (1). It is also used as a semiconductor in electronics.
When gallium solidifies, it can expand up to 3.1% in volume. Storage in glass or metal containers should therefore be avoided; if the temperature were to drop, the container would rupture upon solidification. If too much force is applied, gallium may fracture conchoidally (smooth, curved surfaces, without any natural planes of separation) (2).
Sources:
(1) Gallium: Het element. (2016, November 30). InfoNu.
https://wetenschap.infonu.nl/scheikunde/154971-gallium-het-element.html
(2) Wikipedia contributors. (2021, January 21). Gallium. Wikipedia.
https://en.wikipedia.org/wiki/Gallium
Because it rarely appears in high concentration, germanium was discovered comparatively late in the history of chemistry. In 1869, founder of the periodic table, Dmitri Mendeleev already predicted its existence and some of its properties from its position on his periodic table, and called the element ekasilicon (1). It was German chemist Clemens Winkler who found the new element nearly two decades later, in 1886, along with silver and sulfur. Winkler named the element after his country, Germany. The main application of germanium today is as a carrier material for LEDs, laser diodes and solar cells. You can also find it in infrared optics. Germanium oxide exhibits the unusual property of having a high refractive index for visible light, but transparency to infrared light. This is why it is used a lot in infrared optics (2), wide-angle camera lenses and objective lenses for microscopes (3).
Sources
(1) Wikipedia contributors. (2020t, December 2). Germanium. Wikipedia.
https://en.wikipedia.org/wiki/Germanium
(2) Periodiek systeem - informatie over alle elementen - VNCI. (n.d.-c). C3 Centrum
JongerenCommunicatie Chemie. Retrieved 4 February 2021, from
https://periodieksysteem.com/element/germanium
(3) Germanium - Element information, properties and uses | Periodic Table. (n.d.). Royal
Society of Chemistry. Retrieved 4 February 2021, from https://www.rsc.org/periodic
table/element/32/germanium
Arsenic is a metalloid and occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. (1) Arsenic and its compounds are used in the production of pesticides, treated wood products, herbicides, and insecticides. However, these applications are declining with the increasing recognition of the toxicity of arsenic. Its toxicity is not a new finding; humans have known for hundreds, if not thousands of years that arsenic is poisonous. From the time of the Roman Empire all the way to the Victorian era, arsenic was considered the "king of poisons" as well as the "poison of kings." (2) The name is thought to come from 'arsenikon', the Greek name for the yellow pigment orpiment. (3) The word is also related to the Greek word "arsenikos," meaning "masculine" or "potent.".
Arsenic is a naturally occurring element in the Earth's crust (20th most abundant) and is present at high levels in the groundwater of several countries. However, due to its toxicity, arsenic-contaminated water used for drinking, food preparation and irrigation threatens the health of millions of people worldwide. Within a few years of exposure to arsenic-contaminated water, arsenical skin lesions appear, often leading to skin cancer. This is an especially concerning health problem in populations living along river floodplains of South and Southeast Asia that rely on shallow groundwater wells for drinking water and irrigation, which has resulted in the so-called “worst mass poisoning of human population in history”. (4) Researchers at Radboud University have identified a methane-mediated mechanism for arsenic mobilization. They found that “methane functions as an electron donor for methanotrophs, triggering the reductive dissolution of arsenic-bearing iron(III) minerals, increasing the abundance of genes related to methane oxidation, and ultimately mobilizing arsenic into the water.” (5) As methane has a common presence in arsenic-contaminated aquifers, the researchers suggest that this “methane-driven arsenic mobilization may contribute to arsenic contamination of groundwater on a global scale.” (5)
Sources
(1) Wikipedia contributors. (2021c, January 28). Arsenic. Wikipedia.
https://en.wikipedia.org/wiki/Arsenic
(2) Pedersen, T. (2016, July 28). Facts About Arsenic. Livescience.Com.
https://www.livescience.com/29522-arsenic.html
(3) Arsenic - Element information, properties and uses | Periodic Table. (n.d.). Royal Society
of Chemistry. Retrieved 4 February 2021, from https://www.rsc.org/periodic
table/element/33/arsenic
(4) Smith, A. H., Lingas, E. O. & Rahman, M. Contamination of drinking-water
by arsenic in Bangladesh: a public health emergency. Bull. World Health
Organ. 78, 1093–1103 (2000).
(5) Glodowska, M., Stopelli, E., Schneider, M. et al. Arsenic mobilization by anaerobic iron-
dependent methane oxidation. Commun Earth Environ 1, 42 (2020).
https://doi.org/10.1038/s43247-020-00037-y
Selenium was discovered by chemist Jöns Jacob Berzelius in Stockholm in 1817. He had shares in a sulfuric acid works and was interested in the red-brown sediment which collected at the bottom of the chambers. The element is a semi-metal that can exist in two forms: as a silvery metal or as a red powder. (1) It is named after Selene, the Greek goddess of the moon, because the element was found together with tellurium, named after the Latin ‘tellus’, meaning Earth. (2) Selenium has photovoltaic properties, which convert light directly into electricity. In addition, selenium is also photoconductive; under the influence of light, the electrical conductivity increases. It is therefore useful in photocells, solar cells and photocopiers.
Selenium is an essential trace element for humans. Our bodies contain about 14 milligrams. Too little selenium can cause health problems, but too much is also dangerous. Researchers at Radboudumc found that selenium may play a role in whether or not prostate cancer will spread. Their analysis showed that selenium changes the activity of certain genes implicated in the epithelial-to-mesenchymal transition (EMT) in the prostate, a process linked to cancer progression. Selenium seems to have an inhibitory effect on this EMT process. (3)
Sources
(1) Selenium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society
of Chemistry. Retrieved 4 February 2021, from https://www.rsc.org/periodic
table/element/34/selenium
(2) Wikipedia-bijdragers. (2021, January 20). Seleen. Wikipedia.
https://nl.wikipedia.org/wiki/Seleen
(3) Kok, D. E. G., Kiemeney, L. A. L. M., Verhaegh, G. W., Schalken, J. A., van Lin, E. N. J. T., Sedelaar, J.
P. M., Witjes, J. A., Hulsbergen - van de Kaa, C. A., van ’t Veer, P., Kampman, E., & Afman, L.
A. (2017). A short-term intervention with selenium affects expression of genes implicated in
the epithelial-to-mesenchymal transition in the prostate. Oncotarget, 8(6), 10565–10579.
https://doi.org/10.18632/oncotarget.14551
Bromine is one of the few elements which is liquid at standard temperature and pressure (STP). Elemental bromine is very reactive and thus does not occur free in nature, but in salts similar to table salt. Its name was derived from the Ancient Greek βρῶμος, meaning "stench", referring to its sharp and unpleasant smell. At high temperatures, organobromine compounds (organic compounds that contain carbon bonded to bromine) easily dissociate to yield free bromine atoms, a process that stops free radical chemical chain reactions. This effect makes organobromine compounds useful as fire retardants, which is how more than half the bromine produced worldwide is used. The same ability causes volatile organobromine compounds to dissociate under ultraviolet sunlight, which releases free bromine atoms in the atmosphere, causing ozone depletion. (1) As a result, many organobromide compounds are no longer used.
Researchers at Radboud University have used bromine to produce graphene, a material which consists of a single layer of carbon atoms (learn more about graphene under ‘Carbon’). The researchers produced suspensions of graphene sheets by combining solution-based bromine intercalation and mild sonochemical exfoliation. (2)
Sources
(1) Wikipedia contributors. (2021d, February 2). Bromine. Wikipedia.
https://en.wikipedia.org/wiki/Bromine
(2) Widenkvist, E., Boukhvalov, D. W., Rubino, S., Akhtar, S., Lu, J., Quinlan, R. A., Katsnelson,
M. I., Leifer, K., Grennberg, H., & Jansson, U. (2009). Mild sonochemical exfoliation of
bromine-intercalated graphite: a new route towards graphene. Journal of Physics D:
Applied Physics, 42(11), 112003. https://doi.org/10.1088/0022-3727/42/11/112003
Krypton is a colourless, odourless and tasteless noble gas, which is why its name comes from the Ancient Greek: κρυπτός, Romanized: kryptos, meaning ‘hidden’. Like other noble gases, krypton is used in lighting and photography. Krypton light has many spectral lines, and krypton plasma is useful in bright, high-powered gas lasers, each of which resonates and amplifies a single spectral line. From 1960 to 1983, the official length of a meter was defined by the 606-nanometer wavelength of the orange spectral line of krypton-86. (1) Although krypton is rare and inert, it appears to be able to form connections with a few other elements. Oxygen isn't one of them – the mythical ‘kryptonite’ only exists in Superman stories. (2)
Sources
(1) Wikipedia contributors. (2021a, January 11). Krypton. Wikipedia.
https://en.wikipedia.org/wiki/Krypton
(2) Periodiek systeem - informatie over alle elementen - VNCI. (n.d.-d). C3 Centrum
JongerenCommunicatie Chemie. Retrieved 4 February 2021, from
https://periodieksysteem.com/element/krypton
It seems fair to call rubidium hard to handle: it ignites spontaneously in air and reacts violently with water. In nature, it is only found as an impurity in other minerals. Commercial usage is limited due to its high price and limited supply(1). But wait: it does get better. Rubidium’s name comes from the Latin word ‘rubidus’, which refers to the deep red colour of its emission spectrum. This quality is sometimes exploited in fireworks, resulting in a purple blast(2). Furthermore, rubidium can be used in atomic clocks and it has isotopes that can locate brain tumours(3). Rubidium is also useful when dating – not people, but rocks. This method, rubidium–strontium dating, has been used extensively to determine the age of lunar rocks and meteorites. It builds on the bizarre half-life of one of rubidium’s natural isotopes. Given some quantity of this isotope, it would take 49 billion years before only half is left due to radioactive decay. That is three times as long as the estimated age of the universe(4)!
Sources
(1) Rubidium | chemical element. (n.d.). Encyclopedia Britannica. Retrieved July 29, 2020, from https://www.britannica.com/science/rubidium
(2) Wikipedia contributors. (2020c, July 24). Rubidium. Wikipedia. Retrieved July 29, 2020, from https://en.wikipedia.org/wiki/Rubidium
(3) Rubidium - Element information, properties and uses | Periodic Table. (n.d.). www.rsc.org. Retrieved July 29, 2020, from https://www.rsc.org/periodic-table/element/37/rubidium
(4) Wikipedia contributors. (2020a, June 6). Rubidium–strontium dating. Wikipedia. Retrieved July 29, 2020, from https://en.wikipedia.org/wiki/Rubidium%E2%80%93strontium_dating
Strontium is a highly reactive element with chemical properties similar to its neighbours calcium and barium. Although it is quite common in nature, it only appears in salts due to its extreme reactivity with oxygen and water. Strontium’s applications are quite limited: former uses involve television sets and sugar production, nowadays you may see it light up the air in red fireworks. Still, strontium has some remarkable scientific uses. It behaves similar to calcium, so it is incorporated in small portions of isotopes in human bones. The exact proportions differ depending on location. This helps scientists in determining ancient migration patterns of our ancestors(1). It tells even more: Strontium analysis of the remains found in a gladiator cemetery indicated that gladiators followed a heavily plant-based diet of barley, beans and dried fruits(2). Another scientific face of strontium concerns nuclear power. The radioactive isotope strontium-90 is a by-product of nuclear reactors and it is released during nuclear plant accidents, with high health risks. This for example happened during the 1986 disaster in Chernobyl. On the other hand, it is one of the best high-energy beta-emitters known. Finally, in case you were wondering what gave strontium its curious name: it is named after the Scottish village where it was discovered, called Strontian(3).
Sources
(1) Wikipedia contributors. (n.d.). Strontium. Wikipedia. Retrieved August 7, 2020, from https://en.wikipedia.org/wiki/Strontium
(2) Rupp, R. (2014, November 11). Dinner With the Gladiators: Beans and Ashes. National Geographic. Retrieved August 7, 2020, from https://www.nationalgeographic.com/culture/food/the-plate/2014/11/11/dinner-with-the-gladiators-beans-and-ashes/
(3) Strontium - Element information, properties and uses | Periodic Table. (z.d.). www.rsc.org. Retrieved August 7, 2020, from https://www.rsc.org/periodic-table/element/38/strontium
Yttrium is a silvery-metallic transition metal and is almost always found in combination with lanthanide elements in rare-earth minerals, never as a free element. (1)
Yttrium was discovered in the late 18th century, but only the past few decades this soft, silvery metal found widespread use in chemistry, physics, computer technology, energy, medicine and other fields. It often serves as an additive in alloys, it increases the strength of aluminium and magnesium alloys. (2) Its name derives from the location of its discovery; a quarry near Ytterby, a small town in Sweden. (3)
Sources
(1) Wikipedia contributors. (2021k, February 23). Yttrium. Wikipedia.
https://en.wikipedia.org/wiki/Yttrium
(2) Yttrium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of
Chemistry. Retrieved 13 April 2021, from https://www.rsc.org/periodic
table/element/39/yttrium
(3) Facts About Yttrium. (2018, August 24). Live Science. https://www.livescience.com/34564
yttrium.html
Zirconium is a lustrous, grey-white, strong transition metal. It is named after the mineral Zircon, a gemstone, which comes in blue, yellow, green, brown, orange, red and occasionally purple varieties. The word comes from the Persian "zargun" or gold colour. It has been used in jewelry and other decoration for centuries. (1)
Zirconium is highly resistant to corrosion and is therefore often used in corrosive environments; zirconium alloys can be found in pipes, fittings and heat exchangers. In the 1940s, it became an important material for nuclear energy applications. In nuclear reactors, zirconium is used for cladding fuel rods, for alloying with uranium, and for reactor-core structures because of its unique combination of properties. Zirconium has good strength at elevated temperatures, resists corrosion from the rapidly circulating coolants, does not form highly radioactive isotopes, and withstands mechanical damage from neutron bombardment. (2)
Sources
(1) Ross, R. (2017, January 26). Facts About Zirconium. Live Science.
https://www.livescience.com/34610-zirconium.html
(2) The Editors of Encyclopaedia Britannica. (n.d.). Zirconium | chemical element. Encyclopedia
Britannica. Retrieved 13 April 2021, from https://www.britannica.com/science/zirconium
Niobium is a light grey, crystalline, and ductile transition metal, with a similar hardness of titanium. It is often used in alloys including stainless steel, as it improves the strength of the alloys, especially at low temperatures. Niobium alloys are used in jet engines, rockets, beams and girders for buildings and oil and gas pipelines.(1)
The element was discovered in 1801 by Charles Hatchett, an English scientist who found it in an American ore that had been sent to England more than a century earlier by John Winthrop the Younger, the first governor of the state of Connecticut. The ore, called columbite, had been part of the Hans Sloane collection of the British Museum when Hatchett found it. He reported a new element similar to tantalum and named it columbium. (2) In the decades following his discovery, there was some confusion about the element; some scientists claimed it wasn’t a new element, but that tantalum and columbium were identical. In 1846, the German chemist Heinrich Rose independently discovered an element in tantalum ores, which he named niobium. This name was derived from ‘Niobe’, meaning daughter of Tantalus in Greek mythology (after whom tantalum is named). Years later, a series of scientific findings clarified that niobium and columbium were the same element, and that it was indeed an element that was similar, but independent from tantalum. For a century both names were used interchangeably. It wasn’t until 1949 that the name niobium was adopted internationally, although the name columbium is still used in the United States. (3)
Sources
(1) Niobium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of
Chemistry. Retrieved 15 April 2021, from https://www.rsc.org/periodic
table/element/41/niobium
(2) Niobium Element Facts. (n.d.). Chemicool. Retrieved 15 April 2021, from
https://www.chemicool.com/elements/niobium.html
(3) Wikipedia contributors. (2021n, April 14). Niobium. Wikipedia.
https://en.wikipedia.org/wiki/Niobium
Molybdenum is a silvery-white, ductile metal that is highly resistant to corrosion. (1) It has one of the highest melting points of all pure elements. As a transition metal, molybdenum easily forms compounds with other elements and is not found free in nature, but can be recovered as a by-product of copper or tungsten mining. Molybdenum is mainly used commercially in the production of alloys, where it is added to increase hardness, strength, electrical conductivity and resistance to wear and corrosion. The name molybdenum is derived from Neo-Latin molybdaenum, which is based on Ancient Greek ‘Μόλυβδος’ (molybdos), meaning lead, since its ores were confused with lead ores. (1) Molybdenum minerals have been known throughout history, the element itself was discovered in 1778 by Carl Wilhelm Scheele.
It is also a micronutrient essential for life and present in dozens of enzymes. An example of an important enzyme, is nitrogenase, which allows nitrogen in the atmosphere to be taken up and transformed into compounds that bacteria, plants, animals and humans use to synthesize proteins. (2)
Researchers at Radboud University discovered that that the iron in metal implants can activate drugs such as antibiotics. A grooved implant alloy consisting of cobalt, nickel, chromium and molybdenum, revealed pronounced glycosidic activity, which was is suitable for the conversion of various prodrugs, such as anti-infection, pro-healing, anti-cancer, and anti-inflammation. (3)
Sources
(1) Wikipedia contributors. (2021l, March 18). Molybdenum. Wikipedia.
https://en.wikipedia.org/wiki/Molybdenum
(2) Pedersen, T. (2018, April 12). Facts About Molybdenum. Live Science.
https://www.livescience.com/34687-molybdenum.html
(3) Innate glycosidic activity in metallic implants for localized synthesis of antibacterial drugs, Marja
Bulte-ter Meer et al., Chem. Commun. 2018-11-29, 1359-7345, DOI: 10.1039/C8CC08737G
Almost all available technetium is produced synthetically. Technetium only occurs naturally as a spontaneous fission product in uranium ore and thorium ore, the most common source, or as a product of neutron capture in molybdenum ores. (1) Dmitri Mendeleev, creator of the periodic table already predicted many of technetium's properties before it was discovered. Mendeleev gave the undiscovered element the provisional name ekamanganese (Em), as he predicted the new element to be below manganese in the periodic table. The element was later named technetium (from the Greek τεχνητός, meaning "Craft or Art”) because it was the first artificially produced element. (1)
Technetium-99m, a metastable isotope with a half-life of six hours (2), emits gamma rays and is used at Radboudumc for cancer diagnoses.
Sources
(1) Wikipedia contributors. (2021n, April 6). Technetium. Wikipedia.
https://en.wikipedia.org/wiki/Technetium
(2) Stewart, D. (2012, October 22). Technetium Element Facts. Chemicool.
https://www.chemicool.com/elements/technetium.html
Ruthenium is a rare transition metal belonging to the platinum group, and the last element of this group to be discovered. Like the other metals of the platinum group, ruthenium is inert to most other chemicals. (1) Small amounts of ruthenium are used to harden transition metals like platinum and palladium. (2) Many new uses are emerging for ruthenium, mostly in the electronics industry for chip resistors and electrical contacts. Ruthenium oxide is used in the chemical industry to coat the anodes of electrochemical cells for chlorine production. Ruthenium is also used in catalysts for ammonia and acetic acid production. Ruthenium compounds can be used in solar cells, which turn light energy into electrical energy.
Researchers at Radboud University discovered that ruthenium-based molecular complexes could play an important role in the replacement of fossil fuels by clean and sustainable solar fuels. One of the most attractive solutions in reducing fossil-fuels are photoelectrochemical cells that utilize water as a source of electrons. However, to achieve this, a heterogeneous water oxidation catalyst is needed. Research by Hans Elemans from the Molecular Nanotechnology group within the Institute for Molecules and Materials (IMM) of Radboud University, has shown that when oligomeric ruthenium complexes are anchored on graphitic materials, they behave as molecular electroanodes that catalyse water oxidation to dioxygen at pH 7 with high current densities. This research provides the basis and principles for the design of robust and efficient hybrid molecular electroanode materials for the oxidation of water to dioxygen, and might also lead to new strategies including other transition metals and other catalytic reactions – bringing us one step closer to replacing fossil fuels with clean and sustainable solar fuels. (3)
Sources
(1) Wikipedia contributors. (2021k, February 21). Ruthenium. Wikipedia.
https://en.wikipedia.org/wiki/Ruthenium
(2) Ruthenium Element Facts. (n.d.). Chemicool. Retrieved 20 April 2021, from
https://www.chemicool.com/elements/ruthenium.html
(3) Hoque, M. A., Gil-Sepulcre, M., de Aguirre, A., Elemans, J. A. A. W., Moonshiram, D., Matheu, R.,
Shi, Y., Benet-Buchholz, J., Sala, X., Malfois, M., Solano, E., Lim, J., Garzón-Manjón, A., Scheu,
C., Lanza, M., Maseras, F., Gimbert-Suriñach, C., & Llobet, A. (2020). Water oxidation
electrocatalysis using ruthenium coordination oligomers adsorbed on multiwalled carbon
nanotubes. Nature Chemistry, 12(11), 1060–1066. https://doi.org/10.1038/s41557-020
0548-7
Rhodium is one of the rarest and most valuable precious metals. It is very corrosion resistant(1). Rhodium is found in platinum or nickel ores together, that’s also how it was discovered in 1803 by William Hyde Wollaston(2). It was named for the rose colour of one of its chlorine compounds.
Rhodium is mostly used as a catalyst in the exhausts of cars and in the chemical industry. It also serves as an alloy for platinum and palladium, to make these metals stronger. You can also find it in the contact points of spark plugs, temperature sensors, and jewellery. The high cost ensures that rhodium is applied only as an electroplate (known as ‘rhodinating’: a coating of rhodium improves appearance and resistance of jewels). Rhodium has also been used for honours or to signify elite status, when more commonly used metals such as silver, gold or platinum were deemed insufficient. In 1979 the Guinness Book of World Records gave Paul McCartney a rhodium-plated disc for being history's all-time best-selling songwriter and recording artist(2).
Sources
(1) Rhodium: Het element. (2017, February 10). InfoNu. Retrieved July 24, 2020, from https://wetenschap.infonu.nl/scheikunde/141070-rhodium-het-element.html
(2) Wikipedia contributors. (n.d.). Rhodium. Wikipedia. Retrieved July 24, 2020, from https://en.wikipedia.org/wiki/Rhodium
Palladium is a rare silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston(1). Palladium does not react with oxygen from the air. When the metal heats up, it is easy to manipulate, while it will harden at a low temperature(2). This makes the metal ideally suited for making jewelry.
Palladium can easily switch between different oxidation states, which makes it a very useful catalyst in chemical reactions. Palladium catalysts are particularly effective in so-called cross-coupling reactions (making of carbon-carbon bonds), which are essential in the process of designing, synthesising and testing new potential medicines. As a result, the Nobel Prize in Chemistry of 2010 was awarded to three researchers (Heck, Suzuki and Negishi) for their research on developing new palladium-catalysed cross-coupling reactions.
Palladium is also often used in dentistry, in watches, and in the production of surgical instruments and electrical circuits. However, the main market for palladium is the automotive sector(3), because it is used in catalytic converters, which convert as much as 90% of harmful exhaust gases into less noxious substances(1).
Sources
(1) Wikipedia contributors. (2020b, June 23). Palladium. Wikipedia. Retrieved August 7, 2020, from https://en.wikipedia.org/wiki/Palladium
(2) Wikipedia-bijdragers. (2019, December 14 ). Palladium (element). Wikipedia. Retrieved August 7, 2020, from https://nl.wikipedia.org/wiki/Palladium_(element)
(3) Blekemolen, J. (2020, July 3). De 5 beste palladium aandelen volgens beleggingsspecialist Wubbe Bos. Online Broker LYNX. Retrieved August 7, 2020, from https://www.lynx.nl/kennis/artikelen/top-5-palladium-aandelen-futures-etfs/
Silver is a soft, white, shimmering transition metal and exhibits the highest thermal conductivity, reflectivity and electrical conductivity of any metal(1). It is valued for its decorative beauty, and has long been used for in the manufacture of coins, ornaments, and jewelry(2). Ornaments and decorations date back as far as 4000 BC. Gold and silver were already used as money in Greek communities throughout the Mediterranean Sea and Asia Minor (now known as Turkey) around 800 BC. Silver is also used as a catalyst because of its unique ability to convert ethylene into ethylene oxide, the originator of many organic compounds(2). Silver is one of the noblest – or least chemically reactive – transition elements.
Tzu-Chao Hung, PhD student at Scanning Probe Microscopy department at Radboud University, is using silver to research quantum emitters by the scanning tunnelling microscopy induced light emission. The scanning tunnelling microscope (STM) is a measuring instrument at the Institute for Molecules and Materials, used to examine fundamental physics on the atomic scale. “By utilising this technique, we are able to investigate the optical properties of the quantum emitters down to atomic scale.”, Tzu-Chao Hung explains. “But because the light is coming from a single molecule, the photon intensity is relatively low, as you can expect. Noble metals such as silver are used to enhance the optical signal; a silver cluster terminated STM tip amplifies the signal by almost up to 1000 times.”
Sources
(1) Wikipedia contributors. (n.d.). Silver. Wikipedia. Retrieved July 29, 2020, from https://en.wikipedia.org/wiki/Silver
(2) Hoffmann, J. E. (n.d.). silver | Facts, Properties, & Uses. Encyclopedia Britannica. Retrieved July 29, 2020, from https://www.britannica.com/science/silver

Cadmium was discovered in 1817 simultaneously by German chemists Friedrich Strohmeyer and Karl Samuel Leberecht Hermann, as an impurity in zinc carbonate. Cadmium is so soft you could cut with a knife. Although that might not be such a good idea, as it is one of the most toxic heavy metals (like lead and mercury). Cadmium occurs in most zinc ores and is industrially extracted as a by-product of zinc production(1). In the past, cadmium was used as a corrosion-resistant plating on steel. In old television sets, compounds of cadmium were used as red, orange and yellow pigments. But the use of cadmium has gradually been reduced in consumer items, because it is poisonous and known to cause birth defects and cancer(2). It is specifically listed in the European Restriction of Hazardous Substances(3). Approximately 80% of cadmium currently produced is used in rechargeable nickel-cadmium batteries. However, they are gradually being phased out and replaced with nickel-metal hydride batteries(2). Now, almost 10% of cadmium is extracted from recycled batteries(4).
Helium–cadmium lasers are a common source of blue-ultraviolet laser light and can be used in in fluorescence microscopes and laboratory experiments.
Sources
(1) Wikipedia contributors. (n.d.). Cadmium. Wikipedia. Retrieved July 29, 2020, from https://en.wikipedia.org/wiki/Cadmium
(2) Cadmium - Element information, properties and uses | Periodic Table. (z.d.). Royal Society of Chemistry. Retrieved July 29, 2020, from https://www.rsc.org/periodic-table/element/48/cadmium
(3) Morrow, H. (2010). "Cadmium and Cadmium Alloys". Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons. pp. 1–36. doi:10.1002/0471238961.0301041303011818.a01.pub3. ISBN 978-0-471-23896-6.
(4) Cadmium: het element. (2017, February 24). InfoNu. Retrieved July 29, 2020, from https://wetenschap.infonu.nl/scheikunde/137661-cadmium-het-element.html
Indium was discovered in 1863 by German chemists Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods. Reich, who was colour-blind, hired Richter as an assistant for detecting the coloured spectral lines in zinc chloride. Searching for thallium, they were expecting to find green spectrum lines belonging to thallium. Instead, they (or more accurately; only the colour-seeing Richter) saw a bright indigo-blue line(1). Such a colour had not been previously found, so they hypothesised they had found a new element, which they called indium – a reference to its indigo colour. Indium is a component in zinc sulfide ores and is produced as a by-product of zinc refinement(2). Its first large-scale industrial application was as a coating in high-performance aircraft engines during World War II, to protect them against damage and corrosion(2).
Indium has no metabolic role in any organism. It can be toxic to the kidney when injected, but that’s about as far as it can go: even if you accidentally inhale or eat indium compounds (please don’t!), they wouldn’t be absorbed upon ingestion and are only moderately absorbed on inhalation.
Sources
(1) Gagnon, S. (n.d.). It’s Elemental - The Element Indium. JLab Science Education. Retrieved July 29, 2020, from https://education.jlab.org/itselemental/ele049.html
(2) Wikipedia contributors. (n.d.). Indium. Wikipedia. Retrieved July 29, 2020, from https://en.wikipedia.org/wiki/Indium
Tin, like indium, is soft enough to be cut without much force. The metal lets out a so-called ‘tin-cry’ when bent, as a result of sliding tin crystals reforming; this trait is shared by indium, cadmium, and frozen mercury(1). Another notable phenomenon is the 'tin plague'. This is a change in the material structure of tin at low temperatures. The metal pulverises and loses its strength and firmness. For example, the extreme cold weather conditions destroyed the tin buttons on the uniforms of Napoleon's army during the occupation of Moscow in 1812(3).
Tin is resistant to sea and fresh water. Due to its corrosion resistance and inert behaviour in contact with food, conservation cans are made from tin-plated steel sheets. Tin can be highly polished and is mostly used as a protective coat for other metals. Chances are that you yourself even worked with tin: tin is the main element in solder, the low melting substance used in soldering(2).
Tin is used in research at the High Field Magnet Laboratory at Radboud University. Sanne Kristensen, PhD student Physics, examines fundamental properties of materials, especially the behaviour of electrons in materials under the influence of a strong magnetic field. “In order to measure the electronic properties of materials, it is important to make electronic contact between your material and the measuring equipment. We do this by soldering the contacts with tin. Tin has a melting temperature of approximately 230 degrees Celsius. We heat the tin with a soldering iron until it melts and fuse the tin to the two components that we want to contact electronically. Since tin is a metal, it can transfer the electrons from our material to the wires or electronic components which we use to conduct our experiment”.
Sources
(1) Wikipedia contributors. (n.d.). Tin. Wikipedia. Retrieved July 29, 2020, from https://en.wikipedia.org/wiki/Tin
(2) Het element tin: Eigenschappen en toepassingen. (2017, November 23). InfoNu. Retrieved July 29, 2020, from https://wetenschap.infonu.nl/techniek/112087-het-element-tin-eigenschappen-en-toepassingen.html
(3) Periodiek systeem - informatie over alle elementen - VNCI. (n.d.). Centrum JongerenCommunicatie Chemie. Retrieved July 29, 2020, from https://periodieksysteem.com/element/tin
Antimony compounds have been known since ancient times, in powdered form it was used as medicine and cosmetics. As a lustrous gray metalloid with many different compounds in over a hundred different minerals, it took some time before it was identified as an element; early descriptions of antimony show it has been falsely identified as lead or the sulfide mineral stibnite. The name antimony can be traced back to this abundance of allotropic forms; the Greek words ‘anti’ and ‘monos’ mean “a metal not found alone”. Now, antimony is mainly used as an alloy with lead and tin. (1) One of the largest applications is as lead antimony plates in lead-acid batteries, and antimony is increasingly used in microelectronics. Antimony compounds are used as catalysts for PET production for plastic water bottles. Because antimony compounds can be highly toxic, this has raised concerns about whether the use of antimony in plastic water bottles is safe. Studies have shown that longer the liquid is in the bottle, the higher the level of contamination, (2) although reports show that these levels are still within drinking water guidelines.
Sources
(1) Wikipedia contributors. (2021i, February 16). Antimony. Wikipedia.
https://en.wikipedia.org/wiki/Antimony
(2) Sanderson, K. (n.d.). Toxic risk in bottled water? Chemistry World. Retrieved 17 February
2021, from https://www.chemistryworld.com/news/toxic-risk-in-bottled
water/3004027.article
Tellurium is a silvery white semi metallic element with properties intermediate between those of metals and nonmetals. It is mostly found as compounds of metals such as copper, lead, silver or gold. Tellurium was isolated before it was even known to be an element. In 1782, an Austrian mineralogist was working with an ore known as German gold, from which he obtained a material that, despite numerous attempts, he failed to analyse. He fittingly called it ‘metallum problematicum’. Years later, in 1798, a German chemist established this material was an element, and named it after man’s “heavenly body”; Tellus, meaning Earth. (1) Nowadays, the primary commercial use of tellurium is copper and steel alloys, where it improves machinability. An upcoming application of tellurium is in cadmium telluride (CdTe) solar panels; tellurium demonstrates some of the greatest efficiencies for solar cell electric power generators. (2) Tellurium is considered a ‘technology-critical element’; a chemical element that is important to emerging technologies, in much higher demand than in the past, and in scarce supply relative to demand. Tellurium has no biological function, although fungi can use it in amino acids such as tellurocysteine and telluromethionine.
Sources
(1) Brasted, R. C. (n.d.). Tellurium | chemical element. Encyclopedia Britannica. Retrieved 17
February 2021, from https://www.britannica.com/science/tellurium
(2) Wikipedia contributors. (2021c, January 15). Tellurium. Wikipedia.
https://en.wikipedia.org/wiki/Tellurium
Amidst the Napoleonic wars, the French chemist Bernard Courtois was trying to manufacture saltpetre (an essential element for gunpowder) by exposing decaying organic matter (seaweed ashes, in this case) with sulfuric acid. In the process a cloud of purple vapour rose, which condensed to dark crystals on cold surfaces(1). Not knowing what it was, he gave it the uninspired but slightly mysterious name ‘substance X’. Two years later, in 1813, the British chemist Sir Humphry Davy recognized substance X as an element similar to chlorine; and suggested the name iodine from the Greek word ‘īṓdēs’, which means “violet coloured”.
Iodine is solid at standard conditions, and melts to form a deep violet liquid at 114 °C, and boils to a violet gas at 184 °C(2). In some instances when heated, it can transition directly from solid to gas state, resulting in the misconception that iodine does not exist in liquid form.
“Iodine is an integral component of the thyroid hormone and mediates its effects on brain development.”, Sharon Kolk explains, associate professor in Neurobiology at Radboud University. It is thus an essential dietary mineral for neurodevelopment, especially in offspring and toddlers. A deficit of iodine affects about two billion people and is the leading preventable cause of intellectual disabilities.
Iodine is used as a disinfectant, and in combination with silver (silver iodide), the element has many applications, from photography to air conditioning(3). Adding silver iodide to clouds results in massive rain showers. In 1952, a weather control experiment was conducted in Devon in Great Britain, which accidentally led to severe flooding in the lowland Exmoor area. Thousands of kilograms of silver iodide are still used every year for this technique, called cloud seeding(2).
Sources
(1) Christe, K., & Schneider, S. (n.d.). Iodine | chemical element. Encyclopedia Britannica. Retrieved July 30, 2020, from https://www.britannica.com/science/iodine
(2) Wikipedia contributors. (2001, May 17). Iodine. Wikipedia. Retrieved July 30, 2020, from https://en.wikipedia.org/wiki/Iodine
(3) Thomas, L. (n.d.). Het periodiek systeem: Jodium. Wetenschap in Beeld. Retrieved July 30, 2020, from https://wibnet.nl/natuurkunde/periodiek-systeem/het-periodiek-systeem-jodium

Not only is cesium the most reactive of all metals, it is also one of only five metals that are liquid near room temperature(1). Hence, it responds to your touch: if you held a container of solid cesium in your hand, your body temperature could raise the element’s temperature to its melting point of 28.5 °C and turn it to a liquid. Just remember to not hold caesium directly: then you get a different response (ignition). Cesium gets its name from the Greek word for heavenly blue, which appears in its emission spectrum. However, samples of the element are usually coloured gold, a token of oxygen traces captured in the solid. Ironically, the purer the batch, the less golden it appears(2). Cesium’s most prominent use is time-keeping, or rather, time-defining. One second is officially defined precisely such that the microwave spectral line emitted by the isotope cesium-133 has a frequency of 9,192,631,770 cycles per second(3). Cesium is also a crucial building block in many atomic clocks. These can be so accurate that they neither gain or lose a second in more than 300 million years(4).
Sources
(1) Wikipedia contributors. (n.d.). Cesium. Wikipedia. Retrieved July 31, 2020, from https://en.wikipedia.org/wiki/Caesium
(2) Cesium - Element information, properties and uses | Periodic Table. (n.d.). www.rsc.org. Retrieved July 31, 2020, from https://www.rsc.org/periodic-table/element/55/caesium
(3) cesium | Description, Symbol, Uses, & Facts. (n.d.). Encyclopedia Britannica. Retrieved July 31, 2020, from https://www.britannica.com/science/cesium
(4) Cesium Element Facts. (2012, July 16). Chemicool. Retrieved July 31, 2020, from https://www.chemicool.com/elements/cesium.htm
Barium compounds are extremely heavy, which gives the element its name, based on the Greek barys (heavy). This quality is sometimes used medically in examining patients with digestive complaints. They are given a ‘barium meal’ of the weighty barium sulfate, so the stomach and intestines can be distinguished on an X-ray. Although unpleasant, this procedure is safe as barium sulfate hardly dissolves in water. Barium and its compounds are actually highly toxic. Exposure to higher doses of the metal may cause an irregular heartbeat, anxiety, paralysis, and even death as the heart and lungs fail(1). Barium has been used to commit murder, for example by a 16-year-old in Texas that stole barium acetate from a high school with fatal consequences for the father in 1994(2). The element tells more tales: the barium-bearing mineral barite forms in oceans in proportion to the activity of phytoplankton, the base of the marine food chain. Barite accumulates in marine sediments and is then preserved over millions of years. The result is an archive of ocean history(1). Other uses of barium involve metallurgy, green fireworks, petroleum production(3), and the rare barium mineral benitoite is the official state gem of California, USA(4).
Sources
(1) Barium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of Chemistry. Retrieved August 7, 2020, from https://www.rsc.org/periodic-table/element/56/barium
(2) Stoll, C. (2017, November 7). Facts About Barium. Live Science. Retrieved August 7, 2020, from https://www.livescience.com/37581-barium.html
(3) Barium | chemical element. (n.d.). Encyclopedia Britannica. Retrieved August 7, 2020, from https://www.britannica.com/science/barium
(4) Wikipedia contributors. (n.d.). Barium. Wikipedia. Retrieved August 7, 2020, from https://en.wikipedia.org/wiki/Barium
Lanthanum is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air and is soft enough to be cut with a knife. It is the eponym of the lanthanide series, a group of 15 similar elements between cerium and lutetium in the periodic table. It is also sometimes considered the first element of the 6th-period transition metals, which is why it is displayed here in group 3. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate – hence the name lanthanum, from the Ancient Greek λανθάνειν (lanthanein), meaning "to lie hidden".(1)
In recent years, researchers at Radboud University have discovered a unique biological function of rare-earth elements, or lanthanides, that was previously unknown. Lanthanides have always been regarded as elements that were quite rare, and didn’t play an important role in the biological world. In recent decades, it has become clear that these elements aren’t as rare as we thought, they are more abundant than metals like lead, mercury and gold. Now we are also understanding that they actually play an essential part in various biological processes. Researchers at Radboud University have discovered that many bacteria utilise certain rare earth elements (lanthanides) in their metabolism. Since then, an entirely new area of research has emerged.
“The discovery of bacteria that use lanthanides in their metabolism, came from a bubbling mud pot in Italy”, explains Prof. dr. Huub op den Camp, who is researching the new-found bacteria. “Geologists discovered that geothermally produced gases (CO2, methane, monoxide) associated with these kinds of environments, weren’t all emitted from the mud pool: something was eating them. The answer; methane-eating bacteria.” Lanthanides were shown to play a major role in the second step of methane oxidation, the conversion of methanol into formaldehyde. Previously, the only known enzyme catalysing this reaction, uses calcium as a catalytic cofactor (2). But upon examining the protein structure of the methane-eating bacterium from the volcanic mud pot, the researchers discovered a new lanthanide-dependent methanol dehydrogenase. “This will have major environmental implications as metagenome studies showed these lanthanide containing-type methanol dehydrogenase is much more prominent in nature than the previously known enzymes”. Recently, Huub op den Camp, Arjen Pol and colleagues from an American and German university found that the bacteria can also use actinides instead of their essential lanthanides. They demonstrated that the bacteria are capable of using the radioactive elements americium and curium - which are similar to the lanthanides when it comes to key chemical properties - and grow just as well with these elements. The research group could thus show for the first time that organisms can use these radioactive elements for life processes. These special-skilled bacteria could potentially be used in bioremediation (using living organisms to clean up and restore contaminated environments), or in the separation and recycling of lanthanides and actinides. Such difficult-to-separate mixtures are often found in spent nuclear fuel.
Research on methane- and methanol eating bacteria, specifically methanol dehydrogenase, plays an important role in the Master’s course Genomics of Health and Environment, a collaboration between the departments of Molecular Biology, Human Genetics and Microbiology. Several Master’s internships provide opportunities to join this research, or co-author important scientific articles.
Sources
(1) Wikipedia contributors. (2020o, November 28). Lanthanum. Wikipedia.
https://en.wikipedia.org/wiki/Lanthanum
(2) Pol, A., Barends, T. R. M., Dietl, A., Khadem, A. F., Eygensteyn, J., Jetten, M. S. M., & Op den Camp,
H. J. M. (2013). Rare earth metals are essential for methanotrophic life in volcanic mudpots.
Environmental Microbiology, 16(1), 255–264. https://doi.org/10.1111/1462-2920.12249
(3) Singer, H., Steudtner, R., Klein, A. S., Rulofs, C., Zeymer, C., Drobot, B., Pol, A., Martinez-Gomez, N. C.,
Op den Camp, H. J. M., & Daumann, L. J. (2023). Minor Actinides Can Replace Essential
Lanthanides in Bacterial Life. Angewandte Chemie International Edition, Advance online publication.
https://doi.org/10.1002/anie.202303669
Hafnium is a lustrous, silvery gray, tetravalent transition metal. It chemically resembles zirconium and is found in many zirconium minerals. Although Dmitri Mendeleev predicted the existence of this element in 1869, it wasn’t discovered until 1923 – making it the second-last stable element to be discovered. Hafnium is named after Hafnia, the Latin name for Copenhagen, where it was discovered. (1) As a good absorber of neutrons, it is used to make control rods for nuclear submarines. It is also used in plasma welding torches because of its very high melting point. (2) Hafnium has attracted great scientific and technological interest due to its electronic and superconducting properties. Hafnium oxide is used as an electrical insulator in microchips, and new on-chip uses of hafnium are being researched. For example, research in association with Radboud University has shown that using hafnium oxide thin films in on-chip metasurfaces lead to high-efficiency optical second harmonic generation (SHG) – which is highly desirable for optical sensing, imaging, and quantum photonic systems. (3)
Sources
(1) Wikipedia contributors. (2021r, May 1). Hafnium. Wikipedia.
https://en.wikipedia.org/wiki/Hafnium
(2) Hafnium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of
Chemistry. Retrieved 4 May 2021, from https://www.rsc.org/periodic
table/element/72/hafnium
(3) Qin, J., Huang, F., Li, X., Deng, L., Kang, T., Markov, A., Yue, F., Chen, Y., Wen, X., Liu, S., Xiong, Q.,
Semin, S., Rasing, T., Modotto, D., Morandotti, R., Xu, J., Duan, H., & Bi, L. (2019). Enhanced
Second Harmonic Generation from Ferroelectric HfO2-Based Hybrid Metasurfaces. ACS Nano.
Published. https://doi.org/10.1021/acsnano.8b06308
Tantalum is a rare, very hard, blue-gray, lustrous transition metal that is highly corrosion-resistant. Its chemical properties are very similar to those of niobium, and in the first 45 years after tantalum’s discovery in 1802, there was considerable disagreement whether the substance really was a new element, or just mistaken for the already known niobium (then known as columbium). (1) Tantalum was previously known as tantalium, named after Tantalus, a villain from Greek mythology. Rwanda is the world’s largest extractor of tantalum. Its main use today is in tantalum capacitors in electronic equipment such as mobile phones, DVD players, video game systems and computers. Therefore, it is considered a technology-critical element (an element that is critical to emerging technologies). (2)
Tantalum is being researched at Radboud University for various reasons. Researchers at the department of Condensed Matter Physics used magnetic deflection of tantalum atomic clusters to measure its magnetic moments. (3) And the department of Biomaterials at the Radboud Medical Center has used tantalum oxide in researching techniques to enhance the radiopacity of calcium phosphate cement in vivo. (4)
Sources
(1) Tantalum Element Facts. (2012, September 22). Chemicool.
https://www.chemicool.com/elements/tantalum.html
(2) Wikipedia contributors. (2021r, April 22). Tantalum. Wikipedia.
https://en.wikipedia.org/wiki/Tantalum
(3) Diaz-Bachs, A., Katsnelson, M. I., & Kirilyuk, A. (2018). Kramers degeneracy and relaxation in
vanadium, niobium and tantalum clusters. New Journal of Physics, 20(4), 043042.
https://doi.org/10.1088/1367-2630/aab5ca
(4) Hoekstra, J. W. M., van den Beucken, J. J. J. P., Leeuwenburgh, S. C. G., Bronkhorst, E. M., Meijer,
G. J., & Jansen, J. A. (2013). Tantalum oxide and barium sulfate as radiopacifiers in injectable
calcium phosphate-poly(lactic-co-glycolic acid) cements for monitoringin vivodegradation.
Journal of Biomedical Materials Research Part A, 102(1), 141–149.
https://doi.org/10.1002/jbm.a.34677
Tungsten, or wolfram, is known as one of the toughest things found in nature. It was identified as a new element in 1781 and isolated as a metal in 1783. Tungsten is used in many different ways because it is very strong and durable. The free element is remarkably robust, and has the highest melting and boiling points of all the elements discovered, melting at 3,422 °C and boiling at 5,930 °C. (1) For this reason, tungsten and its alloys are used in many high-temperature applications, such as arc-welding electrodes and heating elements in high-temperature furnaces. One of the most common applications of tungsten used to be the filaments of old-style incandescent light bulbs, but these have been phased because they are not very energy efficient; they produce much more heat than light. (2) Tungsten carbide (made by mixing and heating tungsten and carbon powder) is immensely hard and is used in the metal-working, mining and petroleum industries, for example as cutting and drilling tools. A new ‘painless’ dental drill which spins at ultra-high speeds is also made of tungsten carbide.
Tungsten has been the subject of research at High Field Magnet Laboratory (HFML) at Radboud University, for example by studying its optical properties in valley-dependent phenomena (“valleytronics”), which is very promising for novel opto-electronic applications. (3)
Sources
(1) Wikipedia contributors. (2021t, May 5). Tungsten. Wikipedia.
https://en.wikipedia.org/wiki/Tungsten
(2) Tungsten - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of
Chemistry. Retrieved 5 May 2021, from https://www.rsc.org/periodic
table/element/74/tungsten
(3) Plechinger, G., Nagler, P., Arora, A., Schmidt, R., Chernikov, A., del Águila, A. G., Christianen, P. C.,
Bratschitsch, R., Schüller, C., & Korn, T. (2016). Trion fine structure and coupled spin–valley
dynamics in monolayer tungsten disulfide. Nature Communications, 7(1).
https://doi.org/10.1038/ncomms12715
Rhenium is one of the rarest elements in the Earth's crust, estimated to have a concentration of 1 part per billion (ppb). Rhenium was discovered in 1908 by German chemists and named after the river Rhine(1). It was the second-last stable element to be discovered. Rhenium compounds show a wide variety of oxidation states (the number of electrons that can be lost, thus the hypothetical charge that the atom could have), ranging from −1 to +7.
Nickel-based superalloys of rhenium are used in the combustion chambers, turbine blades, and exhaust nozzles of jet engines(1). These alloys contain up to 6% rhenium, making jet engine construction the largest single use for the element. The second-most important use is as a catalyst: rhenium is an excellent catalyst for hydrogenation and isomerization.
Rhenium has been used at Radboud University in the Scanning Probe Microscopy department (SPM). Rhenium is a superconductor, which means it has absolutely no electrical resistance under a specific temperature. For rhenium this temperature is Tc = 1.7 K. In the SPM group, this property was used to analyse and improve the energy resolution of the Scanning Tunnelling Microscopy setup. The scanning tunnelling microscope (STM) is a measuring instrument at the Institute for Molecules and Materials, used to study fundamental physics on the atomic scale. Just like the resolution of your television screen determines how detailed the image is shown; the energy resolution indicates how many ‘pixels’ of energy the STM can detect. “Ideally, you would measure with such a high resolution that we can see every ‘pixel’, so that we can resolve every small feature in the energy characteristics.”, Elze Knol, PhD student at Scanning Probe Microscopy, explains. “This is impossible to recreate in an experimental setting with a finite temperature, but we try to come as close as possible. That’s where rhenium’s qualities as a superconductor come in; we use it to measure how accurate our resolution is, and how we can improve it. We know that rhenium is in its superconducting state at our measurement temperature (T = 33 mK < Tc). In this case, when measuring the energy spectrum of rhenium, we know that – theoretically – there should be two sharp peaks around E = 0 and in between there should be no signal at all. If the spikes aren’t as sharp as we would expect, for example because they’re blurry or spread out, we know that the energy resolution is not optimal. This is important, because knowing how high the resolution is, tells us how precisely we can resolve small amounts of energy.”
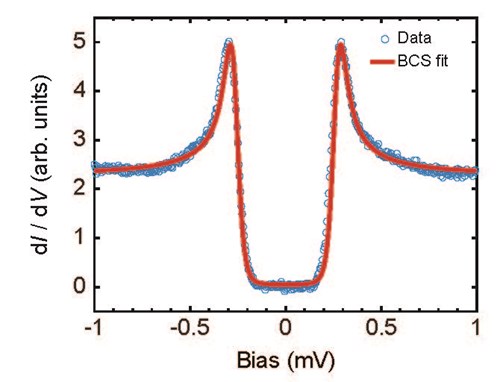
Sources
(1) Wikipedia contributors. (n.d.). Rhenium. Wikipedia. Retrieved July 24, 2020, from https://en.wikipedia.org/wiki/Rhenium
Platinum is one of the least reactive metals and has an exceptional resistance to corrosion(1). Therefore, platinum is often found in its native form, and occurs naturally in sands and rivers. It was first used by pre-Columbian South American natives to produce artifacts. In the 16th century, it was referenced in European writing, but wasn’t seriously investigated until 1748, when Antonio de Ulloa published a report on a new metal of Colombian origin(2).

Click on "Read more" for information about Platinum in research at Radboud University
Sources
(1) Platina: Het element. (2017, Januari 27). InfoNu. Retrieved July 28, 2020, from https://wetenschap.infonu.nl/scheikunde/142138-platina-het-element.html
(2) Wikipedia contributors. (n.d.). Platinum. Wikipedia. Retrieved July 28, 2020, from https://en.wikipedia.org/wiki/Platinum
“Platinum is a metal and commonly known as being crafted in jewelry or used as a catalyst to improve the efficiency of chemical reactions. But it also shows intriguing magnetic properties. For example, it is close to being a ferromagnet, as we know it from iron, cobalt and nickle. Therefore, bringing magnetic material close to platinum, can easily polarize the platinum itself. In addition, platinum is a very heavy element. As a consequence, when it is polarized, its magnetism is more complex than that of a simple ferromagnet, in which the magnetic centers align parallelly. In platinum, they can be twisted and provide a spiral-like magnetic playground”, writes Manuel Steinbrecher, post-doc at Scanning Probe Microscopy.
“In our group, we use platinum as a platform to adsorb single magnetic atoms, like Fe, on the atomically clean platinum surface and investigate their magnetic properties at ultra-low temperatures. We can furthermore move these atoms around on the platinum surface and put them in different positions or create all kinds of larger structures to perfectly control the twisted, magnetic coupling between every atom. As a result of the strong polarization of the platinum, the magnetism of single atom adsorbates is slowed down, resulting in timescales on the order of nanoseconds. Hence, platinum provides a versatile platform to investigate single magnetic atoms and allows to create non-collinear structures with perfectly controlled properties atom-by-atom. Potentially, this allows to create future spintronic devices for information storage and processing with atomic precision.”
Gold is thought to have been formed in supernova explosions and collision of neutron stars, and to have been present in the dust from which the solar system formed. On Earth, gold often occurs in its free native form, as nuggets or grains, in rocks, in veins, and in alluvial deposits(1). As a relatively rare element, gold is a precious metal that has been used in coinage, jewellery, and other arts throughout recorded history. Gold is the earliest recorded metal employed by humans, as small amounts of natural gold have been found in Spanish caves used during the late Palaeolithic period, circa 40,000 BC! Artifacts made of gold first appeared at the beginning of the pre-dynastic period in Egypt and melting was developed quickly after, in the course of the 4th millennium BC. The European exploration of the Americas was greatly fuelled by sightings of gold ornaments made by Native Americans(1).
Gold is used in research at the High Field Magnet Laboratory at Radboud University. Sanne Kristensen, PhD student Physics, examines fundamental properties of materials, especially the behaviour of electrons in materials under the influence of a strong magnetic field. “In order to measure the electronic properties of materials, it is important to make electronic contact between your material and the measuring equipment. Sometimes gold is used to do this; the gold is evaporated into a gas, and then deposited in a thin layer on the material you want to make contact with. Because gold conducts electrons very well and does not oxidise, this method ensures very good electronic contact. Unfortunately, it is also very expensive and time consuming to make contacts with gold, which is why we only use it in cases where it is not possible to solder a contact, like with tin.”
Sources
(1) Wikipedia contributors. (n.d.). Gold. Wikipedia. Retrieved July 24, 2020, from https://en.wikipedia.org/wiki/Gold
Lead is a heavy metal that is denser than most common materials. Lead's high density, low melting point, ductility and relative inertness to oxidation make it useful, combined with its relative abundance and low cost, for extensive use in construction, plumbing, batteries, bullets and shot, weights, solders, pewters, fusible alloys, white paints, leaded gasoline, and radiation shielding(1). Lead was found to be toxic in the 19th century, and its use has been phased out in many applications. It accumulates in soft tissues and bones and can cause neurological disorders.
Lead is used at Radboud University as one of the superconductors to research some quantum mechanical phenomena. When lead is cooled down below 7.2K (-266 °C), it can carry current with zero resistance. When lead is grown in ultrathin film form, it serves as a model system to study the quantum mechanical ‘well problem’. This refers to a type of testing model in which an electronic motion is subjected to quantum confinement, which causes the formation of so-called quantum well states (QWS) in a thin film. These QWS impact film growth and changes the electronic properties of the film. At the Scanning Probe Microscopy department (SPM), researchers use state of the art material synthesis techniques to grow atomically thin films of lead on silicon and other semiconductors. “Our goal is to investigate how quantum well states that are formed in a few atomic layers of lead, affect the superconductivity of lead. This provides a platform to study the transition from 3D to 2D superconductivity (this is reached when the thickness of the superconducting film is comparable to the size of its electron pairs)”, explains Anand Kampalure, Postdoc at the SPM group. “In addition, we also grow a single layer of other material between the lead thin film and semiconductor and study its effect on the superconducting properties. This will help us understand the role of different interfaces and engineer the desired superconducting properties necessary for the next generation devices for faster computation”.
Sources
(1) Wikipedia contributors. (n.d.). Lead. Wikipedia. Retrieved August 16, 2020, from https://en.wikipedia.org/wiki/Lead
Francium is an extremely unstable element and one of the rarest naturally occurring ones. The crust of the entire Earth contains only 20 to 30 grams of francium, so it is no surprise that it was the last element discovered in nature. Its existence was predicted since 1870 and numerous false claims of its discovery were made(1). Finally, it was discovered in 1929 by Marguerite Perey. She was a student of Marie Curie and the first female to be elected to the French Académie des Sciences. During her PhD-exam, she proposed the chemistry-based name catium for the newfound element. One of the examiners mentioned that people might associate this with cats, so instead they based the name on Perey’s native country, France(2). Last but not least, francium is extremely radioactive. Its isotope that survives the longest has a half-life of only 22 minutes. Hence, no one has ever seen francium in bulk and the largest amount ever produced was a cluster of only about 300,000 atoms. Due to this instability and its rarity, francium is not used commercially and only serves research purposes. In fact, scientists even have to do with estimates of usually obvious element properties, like melting and boiling point(1).
Sources
(1) Wikipedia contributors. (n.d.). Francium. Wikipedia. Retrieved July 31, 2020, from https://en.wikipedia.org/wiki/Francium
(2) Francium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of Chemistry. Retrieved July 31, 2020, from https://www.rsc.org/periodic-table/element/87/francium
Roughly a century ago, people were eager to use the newfound element radium in all sorts of applications: Radium was used as an additive in tooth paste, for self-luminous paint in watches(1) and for quackery like ‘Radithor’, which is much like a radioactive counterpart to vitamin water. This ‘triple distilled water’ contained two radium isotopes and was advertised as ‘A Cure for the Living Dead’ - ironically and sadly, it killed at least one of its consumers. This incident was later covered in a Wall Street Journal article with the title "The Radium Water Worked Fine Until His Jaw Came Off"(2). More people have suffered due to naivety surrounding radium’s radioactivity. For example, the so-called Radium Girls were factory workers who painted the dials of luminous watches. They were told to lick their brushes to achieve a fine point, hence they ingested the radium paint. This caused various serious health problems and in many cases even death, as the human body tends to treat the carcinogenic radium as calcium and thus deposits it in the bones. A lawsuit filed by the Radium Girls caught much attention and consequently the health hazards of radium and radioactivity became wildly known(1). The element was discovered in 1898 by Marie Curie and her husband Pierre Curie. Their work with radium did not leave them unscathed either. Curie’s passing, decades later, was almost certainly caused by her exposure to radioactive elements, in particular radium. In fact, to this day her notebooks and papers are kept in lead-lined boxes as they remain radioactive(3). Luckily, there is an upside to radium’s aggressive nature: not only can it destroy healthy tissue, it may also be used to attack cancerous tissue in treatment(1).
Sources
(1) Wikipedia contributors. (n.d.). Radium. Wikipedia. Retrieved August 10, 2020, from https://en.wikipedia.org/wiki/Radium
(2) Wikipedia contributors. (2019, December 28). Radithor. Wikipedia. Retrieved August 10, 2020, from https://en.wikipedia.org/wiki/Radithor
(3) Radium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of Chemistry. Retrieved August 10, 2020, from https://www.rsc.org/periodic-table/element/88/radium
Actinium is a soft, silvery-white radioactive metal and was discovered in 1899 by André Debierne in Paris. (2) Actinium has an eerie blue glow in the dark, due to the element's intense radioactivity. Like most lanthanides and actinides, actinium assumes oxidation state +3 in nearly all its chemical compounds. Actinium is found only in traces in uranium and thorium ores as the isotope 227Ac, which decays with a half-life of 21.772 years, emitting beta and sometimes alpha particles, and 228Ac, which is beta active with a half-life of 6.15 hours. (1) The name actinium is derived from Ancient Greek ἀκτίς (actis), meaning ‘ray’ or ‘radiation’. Due to scarcity, high price and radioactivity, actinium has no significant industrial use. Current applications include a neutron source and an agent for radiation therapy. Actinium has no known biological role and is of course toxic due to its radioactivity.
Actinium-225 is being used in clinical trials at Radboudumc as a next-generation, precision anticancer modality that delivers local alpha therapy to PSMA-expressing cancer cells. (3)
Sources
(1) Wikipedia contributors. (2021f, February 7). Actinium. Wikipedia.
https://en.wikipedia.org/wiki/Actinium
(2) Actinium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society
of Chemistry. Retrieved 9 February 2021, from
https://www.rsc.org/periodictable/element/89/actinium
(3) De Vincentis, G., Gerritsen, W., Gschwend, J. E., Hacker, M., Lewington, V., O’Sullivan, J.
M., Oya, M., Pacilio, M., Parker, C., Shore, N., & Sartor, O. (2019). Advances in
targeted alpha therapy for prostate cancer. Annals of Oncology, 30(11), 1728–1739.
https://doi.org/10.1093/annonc/mdz270
Rutherfordium is an artificially produced element; it is not found in nature and can only be created in a laboratory. It is radioactive; the most stable known isotope, 267Rf, has a half-life of approximately 1.3 hours. Small amounts were first produced in the 1960s, in the Joint Institute for Nuclear Research in the Soviet Union and at the Lawrence Berkeley National Laboratory in California. There was some controversy over the name of the element; the Soviet team and American team each wanted to give the element a different name. It wasn’t until 1997 that the International Union of Pure and Applied Chemistry (IUPAC) established rutherfordium as the official name for the element. (1)
Sources
(1) Wikipedia contributors. (2021f, February 3). Rutherfordium. Wikipedia.
https://en.wikipedia.org/wiki/Rutherfordium
Dubnium is an artificially produced element created by bombarding californium-249 with nitrogen-15 nuclei. (1) It is highly radioactive: the most stable known isotope, dubnium-268, has a half-life of about 28 hours. This has put limits on research on dubnium. Like other synthetical transition metals, dubnium was discovered by the Soviet Joint Institute for Nuclear Research (JINR) in the 1960s, quickly followed by the American Lawrence Berkeley Laboratory in 1970. Again, a long-standing dispute followed about what the official name of the element should be; the Russians called it neilsbohrium, while the Americans called it hahnium, both derived from the names of prominent nuclear scientists. In 1993, an official investigation of both discovery claims resulted in a shared credit for the discovery. The element was named dubnium after the town of Dubna, the site of the JINR. (2)
Sources
(1) Dubnium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society
of Chemistry. Retrieved 9 February 2021, from
https://www.rsc.org/periodictable/element/105/dubnium
(2) Wikipedia contributors. (2021a, January 9). Dubnium. Wikipedia.
https://en.wikipedia.org/wiki/Dubnium
Seaborgium is a synthetic element (created in a laboratory, not found in nature), named after the American nuclear chemist Glenn T. Seaborg. It is radioactive; the most stable known isotope, 269Sg, has a half-life of approximately 14 minutes. In 1974, a few atoms of seaborgium were produced in laboratories in the Soviet Union and in the United States. As other elements produced at that time, the naming of the element was disputed between Soviet and American scientists, and it was not until 1997 that the International Union of Pure and Applied Chemistry (IUPAC) established seaborgium as the official name for the element. It is one of only two elements named after a living person at the time of naming, the other being oganesson. (1)
Sources
(1) Wikipedia contributors. (2021k, March 24). Seaborgium. Wikipedia.
https://en.wikipedia.org/wiki/Seaborgium
Bohrium is a synthetic element (made in a laboratory, not found in nature), named after Danish physicist Niels Bohr. Bohrium does not occur naturally and only a few atoms have ever been made. It was created by the so-called ‘cold fusion’ method. (2) This involved the bombardment of bismuth with atoms of chromium. All known isotopes of bohrium are extremely radioactive; the most stable known isotope is 270Bh with a half-life of approximately 61 seconds, though the unconfirmed 278Bh may have a longer half-life of about 690 seconds. (1)
Sources
(1) Wikipedia contributors. (2021l, April 8). Bohrium. Wikipedia.
https://en.wikipedia.org/wiki/Bohrium
(2) Bohrium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of
Chemistry. Retrieved 12 April 2021, from https://www.rsc.org/periodic
table/element/107/bohrium
Cerium is the most abundant of the rare earth elements (lanthanides) and is the 26th-most abundant element, making up about 0.0046% of the earth's crust. Pure cerium will ignite if it is scratched with a sharp object, but can be safely used in combination with other materials. Cerium is used to make carbon arc lights, for example in the film industry for studio lighting and projector lights. Cerium is also a component of Misch metal, a material that is used to make flints for lighters. (1). Cerium was discovered in 1803 and was named after the dwarf planet Ceres, which was discovered two years earlier. The dwarf planet itself is named after the Roman goddess of agriculture, grain crops, fertility and motherly relationships, Ceres. (2)
In recent years, researchers at Radboud University have discovered a unique biological function of rare-earth elements, or lanthanides, that was previously unknown. Lanthanides have always been regarded as elements that were quite rare, and didn’t play an important role in the biological world. In recent decades, it has become clear that these elements aren’t as rare as we thought, they are more abundant than metals like lead, mercury and gold. Now we are also understanding that they actually play an essential part in various biological processes. Researchers at Radboud University have discovered that many bacteria utilise certain rare earth elements (lanthanides) in their metabolism. Since then, an entirely new area of research has emerged.
“The discovery of bacteria that use lanthanides in their metabolism, came from a bubbling mud pot in Italy”, explains Prof. dr. Huub op den Camp, who is researching the new-found bacteria. “Geologists discovered that geothermally produced gases (CO2, methane, monoxide) associated with these kinds of environments, weren’t all emitted from the mud pool: something was eating them. The answer; methane-eating bacteria”. Lanthanides were shown to play a major role in the second step of methane oxidation, the conversion of methanol into formaldehyde. Previously, the only known enzyme catalysing this reaction, uses calcium as a catalytic cofactor (3). But upon examining the protein structure of the methane-eating bacterium from the volcanic mud pot, the researchers discovered a new lanthanide-dependent methanol dehydrogenase. “This will have major environmental implications as metagenome studies showed these lanthanide containing-type methanol dehydrogenase is much more prominent in nature than the previously known enzymes”.
Research on methane- and methanol eating bacteria, specifically methanol dehydrogenase, plays an important role in the Master’s course Genomics of Health and Environment, a collaboration between the departments of Molecular Biology, Human Genetics and Microbiology. Several Master’s internships provide opportunities to join this research, or co-author important scientific articles.
Sources
(1) Gagnon, S. (n.d.-a). It’s Elemental - The Element Cerium. JLab Science Education. Retrieved 8
December 2020, from https://education.jlab.org/itselemental/ele058.html
(2) Wikipedia contributors. (2020p, December 1). Cerium. Wikipedia.
https://en.wikipedia.org/wiki/Cerium
(3) Pol, A., Barends, T. R. M., Dietl, A., Khadem, A. F., Eygensteyn, J., Jetten, M. S. M., & Op den Camp,
J. M. (2013). Rare earth metals are essential for methanotrophic life in volcanic mudpots.
Environmental Microbiology, 16(1), 255–264. https://doi.org/10.1111/1462-2920.12249
Praseodymium is a soft, silvery, malleable and ductile metal, valued for its magnetic, electrical, chemical, and optical properties. It is too reactive to be found in native form, and pure praseodymium metal slowly develops a green oxide coating when exposed to air. (1) Mischmetal is an alloy containing about 5% praseodymium and is used to make flints for cigarette lighters. Praseodymium is also used in alloys for permanent magnets. (2)
In recent years, researchers at Radboud University have discovered a unique biological function of rare-earth elements, or lanthanides, that was previously unknown. Lanthanides have always been regarded as elements that were quite rare, and didn’t play an important role in the biological world. In recent decades, it has become clear that these elements aren’t as rare as we thought, they are more abundant than metals like lead, mercury and gold. Now we are also understanding that they actually play an essential part in various biological processes. Researchers at Radboud University have discovered that many bacteria utilise certain rare earth elements (lanthanides) in their metabolism. Since then, an entirely new area of research has emerged.
“The discovery of bacteria that use lanthanides in their metabolism, came from a bubbling mud pot in Italy”, explains Prof. dr. Huub op den Camp, who is researching the new-found bacteria. “Geologists discovered that geothermally produced gases (CO2, methane, monoxide) associated with these kinds of environments, weren’t all emitted from the mud pool: something was eating them. The answer; methane-eating bacteria”. Lanthanides were shown to play a major role in the second step of methane oxidation, the conversion of methanol into formaldehyde. Previously, the only known enzyme catalysing this reaction, uses calcium as a catalytic cofactor (3). But upon examining the protein structure of the methane-eating bacterium from the volcanic mud pot, the researchers discovered a new lanthanide-dependent methanol dehydrogenase. “This will have major environmental implications as metagenome studies showed these lanthanide containing-type methanol dehydrogenase is much more prominent in nature than the previously known enzymes”.
Research on methane- and methanol eating bacteria, specifically methanol dehydrogenase, plays an important role in the Master’s course Genomics of Health and Environment, a collaboration between the departments of Molecular Biology, Human Genetics and Microbiology. Several Master’s internships provide opportunities to join this research, or co-author important scientific articles.
Sources
(1) Wikipedia contributors. (2020q, December 3). Praseodymium. Wikipedia.
https://en.wikipedia.org/wiki/Praseodymium
(2) Praseodymium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of
Chemistry. Retrieved 8 December 2020, from
https://www.rsc.org/periodictable/element/59/praseodymium
(3) Pol, A., Barends, T. R. M., Dietl, A., Khadem, A. F., Eygensteyn, J., Jetten, M. S. M., & Op den Camp,
H. J. M. (2013). Rare earth metals are essential for methanotrophic life in volcanic mudpots.
Environmental Microbiology, 16(1), 255–264. https://doi.org/10.1111/1462-2920.12249
Austrian scientist Carl Auer von Welsbach first identified neodymium in 1885. He found it in the substance ‘didymium’, which was discovered in 1841 and incorrectly identified as a new element(1). Neodymium is a soft, bright, silvery white metal. When exposed to air and moisture, neodymium easily forms a thin layer of corrosion(2). But unlike many metal oxide coating, this one does not protect the metal from further oxidation. When oxidised, neodymium reacts quickly to produce pink, purple-blue and yellow compounds. These compounds are commercially used as glass dyes.
Another important use of neodymium is as a component in the alloys used to make high-strength, powerful magnets. A neodymium magnet of a few grams can lift a thousand times its own weight.
Neodymium’s magnetic properties are being researched by physicists at the Scanning Probe Microscopy department (SPM) at Radboud University. The high-precision tunnelling microscopy enables researchers to see the magnetic structure at the atomic scale.
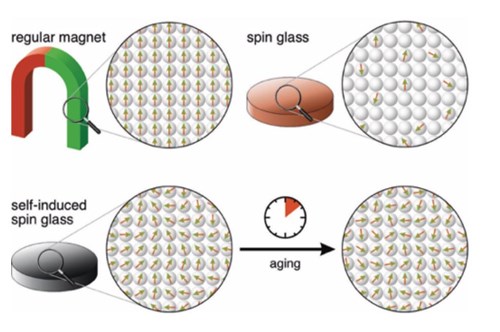
“Recently, we found that neodymium hosts an intriguing new magnetic state of matter, namely ‘self-induced spin glass’ ”, says Umut Kamber, PhD student at SPM. A spin glass is a type of magnetic structure in ‘disordered’ magnets, meaning their atoms spins are not aligned in a regular pattern. “In this structure, the atomic spins of neodymium form whirling patterns that whirl like a helix, but constantly change the exact pattern of the helix.” Essentially, it evolves its own magnetic spins over time. “Such a novel type of spin glass has been predicted in a theoretical model by Prof. Misha Katsnelson. We confirmed the existence of this model and showed how self-induced spin glass looks like in real space.” Understanding this new type of magnetic behaviour refines our understanding of elements on the periodic table, and could eventually pave the way for new materials for artificial intelligence.
Sources
(1) Neodymium Element Facts / Chemistry. (n.d.). Chemicool. Retrieved July 30, 2020, from https://www.chemicool.com/elements/neodymium.html
(2) Wikipedia contributors. (n.d.). Neodymium. Wikipedia. Retrieved July 30, 2020, from https://en.wikipedia.org/wiki/Neodymium
Click on "Read more" to read about research on lanthanides at Radboud University.
In recent years, researchers at Radboud University have discovered a unique biological function of rare-earth elements, or lanthanides, that was previously unknown. Lanthanides have always been regarded as elements that were quite rare, and didn’t play an important role in the biological world. In recent decades, it has become clear that these elements aren’t as rare as we thought, they are more abundant than metals like lead, mercury and gold. Now we are also understanding that they actually play an essential part in various biological processes. Researchers at Radboud University have discovered that many bacteria utilise certain rare earth elements (lanthanides) in their metabolism. Since then, an entirely new area of research has emerged.
“The discovery of bacteria that use lanthanides in their metabolism, came from a bubbling mud pot in Italy”, explains Prof. dr. Huub op den Camp, who is researching the new-found bacteria. “Geologists discovered that geothermally produced gases (CO2, methane, monoxide) associated with these kinds of environments, weren’t all emitted from the mud pool: something was eating them. The answer; methane-eating bacteria”. Lanthanides were shown to play a major role in the second step of methane oxidation, the conversion of methanol into formaldehyde. Previously, the only known enzyme catalysing this reaction, uses calcium as a catalytic cofactor1. But upon examining the protein structure of the methane-eating bacterium from the volcanic mud pot, the researchers discovered a new lanthanide-dependent methanol dehydrogenase. “This will have major environmental implications as metagenome studies showed these lanthanide containing-type methanol dehydrogenase is much more prominent in nature than the previously known enzymes”.
Research on methane- and methanol eating bacteria, specifically methanol dehydrogenase, plays an important role in the Master’s course Genomics of Health and Environment, a collaboration between the departments of Molecular Biology, Human Genetics and Microbiology. Several Master’s internships provide opportunities to join this research, or co-author important scientific articles.
Sources
1 Pol, A., Barends, T. R. M., Dietl, A., Khadem, A. F., Eygensteyn, J., Jetten, M. S. M., & Op den Camp, J. M. (2013). Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environmental Microbiology, 16(1), 255–264. https://doi.org/10.1111/1462-2920.12249
All of Promethium’s isotopes are radioactive. It is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium shows only one stable oxidation state of +3. In 1902 Czech chemist Bohuslav Brauner suggested that there was a then-unknown element with properties intermediate between those of the known elements neodymium (60) and samarium (62). In 1926, two groups (one Italian and one American) claimed to have isolated a sample of element 61, but both "discoveries" were later proven to be false. In 1938, a few radioactive nuclides were produced during a nuclear experiment conducted at Ohio State University, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognised. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name "prometheum" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize "both the daring and the possible misuse of mankind's intellect". (1)
In recent years, researchers at Radboud University have discovered a unique biological function of rare-earth elements, or lanthanides, that was previously unknown. Lanthanides have always been regarded as elements that were quite rare, and didn’t play an important role in the biological world. In recent decades, it has become clear that these elements aren’t as rare as we thought, they are more abundant than metals like lead, mercury and gold. Now we are also understanding that they actually play an essential part in various biological processes. Researchers at Radboud University have discovered that many bacteria utilise certain rare earth elements (lanthanides) in their metabolism. Since then, an entirely new area of research has emerged.
“The discovery of bacteria that use lanthanides in their metabolism, came from a bubbling mud pot in Italy”, explains Prof. dr. Huub op den Camp, who is researching the new-found bacteria. “Geologists discovered that geothermally produced gases (CO2, methane, monoxide) associated with these kinds of environments, weren’t all emitted from the mud pool: something was eating them. The answer; methane-eating bacteria”. Lanthanides were shown to play a major role in the second step of methane oxidation, the conversion of methanol into formaldehyde. Previously, the only known enzyme catalysing this reaction, uses calcium as a catalytic cofactor (2). But upon examining the protein structure of the methane-eating bacterium from the volcanic mud pot, the researchers discovered a new lanthanide-dependent methanol dehydrogenase. “This will have major environmental implications as metagenome studies showed these lanthanide containing-type methanol dehydrogenase is much more prominent in nature than the previously known enzymes”.
Research on methane- and methanol eating bacteria, specifically methanol dehydrogenase, plays an important role in the Master’s course Genomics of Health and Environment, a collaboration between the departments of Molecular Biology, Human Genetics and Microbiology. Several Master’s internships provide opportunities to join this research, or co-author important scientific articles.
Sources
(1) Wikipedia contributors. (2020q, December 1). Promethium. Wikipedia.
https://en.wikipedia.org/wiki/Promethium
(2) Pol, A., Barends, T. R. M., Dietl, A., Khadem, A. F., Eygensteyn, J., Jetten, M. S. M., & Op den Camp,
J. M. (2013). Rare earth metals are essential for methanotrophic life in volcanic mudpots.
Environmental Microbiology, 16(1), 255–264. https://doi.org/10.1111/1462-2920.12249
Samarium is a lanthanide (rare earth metal) with a hardness and density similar to those of zinc. The most important commercial application of samarium is in samarium–cobalt magnets, which have permanent magnetisation, just like neodymium magnets. However, unlike neodymium, samarium compounds can withstand significantly higher temperatures, above 700 °C (1,292 °F), without losing their magnetic properties.
In recent years, researchers at Radboud University have discovered a unique biological function of rare-earth elements, or lanthanides, that was previously unknown. Lanthanides have always been regarded as elements that were quite rare, and didn’t play an important role in the biological world. In recent decades, it has become clear that these elements aren’t as rare as we thought, they are more abundant than metals like lead, mercury and gold. Now we are also understanding that they actually play an essential part in various biological processes. Researchers at Radboud University have discovered that many bacteria utilise certain rare earth elements (lanthanides) in their metabolism. Since then, an entirely new area of research has emerged.
“The discovery of bacteria that use lanthanides in their metabolism, came from a bubbling mud pot in Italy”, explains Prof. dr. Huub op den Camp, who is researching the new-found bacteria. “Geologists discovered that geothermally produced gases (CO2, methane, monoxide) associated with these kinds of environments, weren’t all emitted from the mud pool: something was eating them. The answer; methane-eating bacteria”. Lanthanides were shown to play a major role in the second step of methane oxidation, the conversion of methanol into formaldehyde. Previously, the only known enzyme catalysing this reaction, uses calcium as a catalytic cofactor (2). But upon examining the protein structure of the methane-eating bacterium from the volcanic mud pot, the researchers discovered a new lanthanide-dependent methanol dehydrogenase. “This will have major environmental implications as metagenome studies showed these lanthanide containing-type methanol dehydrogenase is much more prominent in nature than the previously known enzymes”.
Research on methane- and methanol eating bacteria, specifically methanol dehydrogenase, plays an important role in the Master’s course Genomics of Health and Environment, a collaboration between the departments of Molecular Biology, Human Genetics and Microbiology. Several Master’s internships provide opportunities to join this research, or co-author important scientific articles.
Sources
(1) Wikipedia contributors. (2020o, November 25). Samarium. Wikipedia.
https://en.wikipedia.org/wiki/Samarium
(2) Pol, A., Barends, T. R. M., Dietl, A., Khadem, A. F., Eygensteyn, J., Jetten, M. S. M., & Op den Camp,
J. M. (2013). Rare earth metals are essential for methanotrophic life in volcanic mudpots.
Environmental Microbiology, 16(1), 255–264. https://doi.org/10.1111/1462-2920.12249
Europium is the softest lanthanide; you can dent it with your fingernail and cut it with a knife. Europium was discovered in 1901 and is named after the continent of Europe. Compared to other heavy metals, such as mercury, cadmium and lead, Europium is relatively non-toxic. (1) Most applications of europium exploit the phosphorescence (‘glow in the dark’) of europium compounds, for example in old CRT televisions or the luminescence in Euro banknotes. (2) Europium is one of the rarest of the rare earth elements on Earth.
In recent years, researchers at Radboud University have discovered a unique biological function of rare-earth elements, or lanthanides, that was previously unknown. Lanthanides have always been regarded as elements that were quite rare, and didn’t play an important role in the biological world. In recent decades, it has become clear that these elements aren’t as rare as we thought, they are more abundant than metals like lead, mercury and gold. Now we are also understanding that they actually play an essential part in various biological processes. Researchers at Radboud University have discovered that many bacteria utilise certain rare earth elements (lanthanides) in their metabolism. Since then, an entirely new area of research has emerged.
“The discovery of bacteria that use lanthanides in their metabolism, came from a bubbling mud pot in Italy”, explains Prof. dr. Huub op den Camp, who is researching the new-found bacteria. “Geologists discovered that geothermally produced gases (CO2, methane, monoxide) associated with these kinds of environments, weren’t all emitted from the mud pool: something was eating them. The answer; methane-eating bacteria”. Lanthanides were shown to play a major role in the second step of methane oxidation, the conversion of methanol into formaldehyde. Previously, the only known enzyme catalysing this reaction, uses calcium as a catalytic cofactor (3). But upon examining the protein structure of the methane-eating bacterium from the volcanic mud pot, the researchers discovered a new lanthanide-dependent methanol dehydrogenase. “This will have major environmental implications as metagenome studies showed these lanthanide containing-type methanol dehydrogenase is much more prominent in nature than the previously known enzymes”.
Research on methane- and methanol eating bacteria, specifically methanol dehydrogenase, plays an important role in the Master’s course Genomics of Health and Environment, a collaboration between the departments of Molecular Biology, Human Genetics and Microbiology. Several Master’s internships provide opportunities to join this research, or co-author important scientific articles.
Sources
(1) Wikipedia contributors. (2020o, November 14). Europium. Wikipedia.
https://en.wikipedia.org/wiki/Europium
(2) Periodiek systeem - informatie over alle elementen - VNCI. (n.d.-b). Centrum
JongerenCommunicatie Chemie. Retrieved 9 December 2020, from
https://periodieksysteem.com/element/europium
(3) Pol, A., Barends, T. R. M., Dietl, A., Khadem, A. F., Eygensteyn, J., Jetten, M. S. M., & Op den Camp,
J. M. (2013). Rare earth metals are essential for methanotrophic life in volcanic mudpots.
Environmental Microbiology, 16(1), 255–264. https://doi.org/10.1111/1462-2920.12249
Uranium is the element with the highest atomic mass that occurs naturally on Earth. It is quite rare in nature; it occurs in low concentrations of a few parts per million in soil, rocks and water(1). Uranium is extracted from minerals such as urinite (image below).
Uranium is weakly radioactive; all isotopes of uranium are unstable and the half-lives of its naturally occurring isotopes range between 159,200 years and 4.5 billion years. The half-life of the most common isotope uranium-238 is 4.47 billion years(2)! Therefore these radioisotopes are useful in determining the age of the Earth, which is currently estimated to be 4.54 billion years old.

But uranium perhaps became the most famous for its nuclear properties. The isotope uranium-235 is the only naturally occurring isotope which is capable of sustaining a ‘nuclear fission chain reaction’; which means the centre of its atom splits into two or more smaller parts, generating an astonishing amount of energy. In nuclear reactors, the fission process is regulated and the energy is utilised to produce electricity. In nuclear weapons, the fission energy is released all at once to produce a violent explosion(3). Although nuclear fuel is a very dense source of energy, meaning it contains a lot of free energy (millions of times the amount of energy contained in chemical fuels such as gasoline), one of the pressing problems involved in this process is that products of the nuclear fission process are far more radioactive than the original elements, resulting in highly radioactive and long-lasting nuclear waste(4).
Sources
1 Uranium: Het element. (2017, February 19). InfoNu. Retrieved August 10, 2020, from https://wetenschap.infonu.nl/scheikunde/143311-uranium-het-element.html
2 Wikipedia contributors. (n.d.). Uranium. Wikipedia. Retrieved August 10, 2020, from https://en.wikipedia.org/wiki/Uranium
3 Fissile Material Basics. (2012, April 19). Institute for Energy and Environmental Research. Retrieved August 10, 2020, from https://ieer.org/resource/factsheets/fissile-material-basics/
4 Wikipedia contributors. (n.d.). Nuclear fission. Wikipedia. Retrieved August 10, 2020, from https://en.wikipedia.org/wiki/Nuclear_fission
If you recall that the seventh planet of our solar system is Uranus, you probably also remember there once was a ninth planet: Pluto. Although since 2006 planet Pluto is no more, its former status is still commemorated on the periodic table. Plutonium, with atomic number 94, lies two spots to the right of uranium, with atomic number 92. What element lies in between? Of course, that can only be neptunium, named after the heavenly body located between Uranus and Pluto. Just like Neptune, neptunium can hardly be found on earth. In nature it only appears in the tiniest of quantities in uranium ores. More neptunium may be found in the average household, namely as a by-product in smoke detectors. Yet, this too involves only the smallest quantities. That is a good thing probably, as 60 kg of the isotope neptunium-237 could produce a nuclear explosion. Given that every year, more that 50 tonnes of neptunium is created as waste in nuclear reactors, it may come as a surprise that it has not been used in for destructive purposes. The explanation is that the element has no particular advantages over plutonium and enriched uranium(1). This absence of outstanding, handy qualities seems a recurring theme with neptunium: it has no commercial uses(2).
Sources
(1) Neptunium - Element information, properties and uses | Periodic Table. (n.d.). Royal Society of Chemistry. Retrieved August 27, 2020, from https://www.rsc.org/periodic-table/element/93/neptunium
(2) Wikipedia contributors. (n.d.). Neptunium. Wikipedia. Retrieved August 27, 2020, from https://en.wikipedia.org/wiki/Neptunium
As the name would suggest, plutonium was named after the (dwarf) planet Pluto, continuing a tradition that started with uranium (92) and neptunium (93). However, the elements have nothing to do with their planetary namesakes.
Plutonium does not occur naturally, but arises when the isotope uranium-238 captures neutrons emitted by decay of other uranium-238 atoms, a fission process which converts uranium-238 nuclei into plutonium-239. Just like uranium, isotopes of plutonium are also fissile materials, which means they are capable of sustaining a nuclear chain reaction used in nuclear reactors and weapons(1). Producing plutonium was a major part of the Manhattan Project during World War II. This project was initiated to develop the first atomic bomb. The Fat Man bombs used in the bombing of Nagasaki in August 1945, had plutonium cores. Plutonium is radioactive and highly dangerous to handle without protection, because it can accumulate in bones.
The isotope plutonium-238 has a half-life of 87.74 years and emits a large amount of thermal energy, combining high energy radiation with low penetration. This makes it well-suited for electrical power generation in devices that need to go without maintenance for a long time. It is used for example in generators and heater units in long-lasting space probes like Cassini, Voyager, Galileo and New Horizons, and the Curiosity Mars rover(1).
Sources
(1) Wikipedia contributors. (n.d.). Plutonium. Wikipedia. Retrieved July 24, 2020, from https://en.wikipedia.org/wiki/Plutonium
A team of researchers from Radboud University, UC Berkeley, and LMU Munich has discovered that certain bacteria can use radioactive elements, specifically americium and curium, in their metabolism instead of rare earth metals (or lanthanides), challenging the previous belief that lanthanides were indispensable for bacterial metabolism.
Earlier, Prof. dr. Huub op den Camp, Dr. Arjan Pol and colleagues from Radboud University showed that methylotrophs bacteria, which geologists found in a mud pool on an Italian volcano, incorporate lanthanides into a crucial enzyme called lanthanide-dependent methanol dehydrogenase, which enables them to utilize methanol or methane as energy sources (1) (read more under the element "Lanthanum").
Now, an international research group with Huub op den Camp and Arjan Pol from Radboud University, has shown that the bacteria can also incorporate the radioactive materials americium and curium, as they are similar to the lanthanides when it comes to key chemical properties (2). Interestingly, when given a mixture of lanthanides and actinides, the bacteria even showed a preference for americium and curium over some lanthanides. The research group could thus show for the first time that organisms can use these radioactive elements for life processes. These special-skilled bacteria could be used in applications in bioremediation and the separation and recycling of lanthanides and actinides. Such difficult-to-separate mixtures are often found in spent nuclear fuel.
(1) Pol, A., Barends, T. R. M., Dietl, A., Khadem, A. F., Eygensteyn, J., Jetten, M. S. M., & Op den Camp,
H. J. M. (2013). Rare earth metals are essential for methanotrophic life in volcanic mudpots.
Environmental Microbiology, 16(1), 255–264. https://doi.org/10.1111/1462-2920.12249
(2) Singer, H., Steudtner, R., Klein, A. S., Rulofs, C., Zeymer, C., Drobot, B., Pol, A., Martinez-Gomez, N. C.,
Op den Camp, H. J. M., & Daumann, L. J. (2023). Minor Actinides Can Replace Essential
Lanthanides in Bacterial Life. Angewandte Chemie International Edition, Advance online publication.
https://doi.org/10.1002/anie.202303669
A team of researchers from Radboud University, UC Berkeley, and LMU Munich has discovered that certain bacteria can use radioactive elements, specifically americium and curium, in their metabolism instead of rare earth metals (or lanthanides), challenging the previous belief that lanthanides were indispensable for bacterial metabolism.
Earlier, Prof. dr. Huub op den Camp, Dr. Arjan Pol and colleagues from Radboud University showed that methylotrophs bacteria, which geologists found in a mud pool on an Italian volcano, incorporate lanthanides into a crucial enzyme called lanthanide-dependent methanol dehydrogenase, which enables them to utilize methanol or methane as energy sources (1) (read more under the element "Lanthanum").
Now, an international research group with Huub op den Camp and Arjan Pol from Radboud University, has shown that the bacteria can also incorporate the radioactive materials americium and curium, as they are similar to the lanthanides when it comes to key chemical properties (2). Interestingly, when given a mixture of lanthanides and actinides, the bacteria even showed a preference for americium and curium over some lanthanides. The research group could thus show for the first time that organisms can use these radioactive elements for life processes. These special-skilled bacteria could be used in applications in bioremediation and the separation and recycling of lanthanides and actinides. Such difficult-to-separate mixtures are often found in spent nuclear fuel.
(1) Pol, A., Barends, T. R. M., Dietl, A., Khadem, A. F., Eygensteyn, J., Jetten, M. S. M., & Op den Camp,
H. J. M. (2013). Rare earth metals are essential for methanotrophic life in volcanic mudpots.
Environmental Microbiology, 16(1), 255–264. https://doi.org/10.1111/1462-2920.12249
(2) Singer, H., Steudtner, R., Klein, A. S., Rulofs, C., Zeymer, C., Drobot, B., Pol, A., Martinez-Gomez, N. C.,
Op den Camp, H. J. M., & Daumann, L. J. (2023). Minor Actinides Can Replace Essential
Lanthanides in Bacterial Life. Angewandte Chemie International Edition, Advance online publication.
https://doi.org/10.1002/anie.202303669